Introduction to models and their uses Why When



![Why is Quantitative Analysis Important? Toy system: A futile cyle. B-p [A]ss Å A-p Why is Quantitative Analysis Important? Toy system: A futile cyle. B-p [A]ss Å A-p](https://slidetodoc.com/presentation_image_h2/fbf4d238e9b66bb1a07f7fe2796862cc/image-15.jpg)






- Slides: 34

Introduction to models and their uses Why, When, and for What?

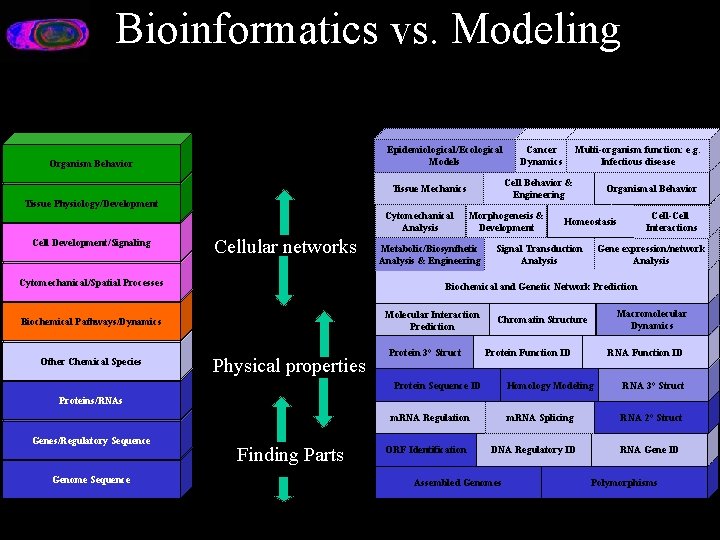
Bioinformatics vs. Modeling Epidemiological/Ecological Models Organism Behavior Tissue Physiology/Development Cell Development/Signaling Cellular networks Cytomechanical/Spatial Processes Morphogenesis & Development Metabolic/Biosynthetic Analysis & Engineering Organismal Behavior Cell-Cell Interactions Homeostasis Signal Transduction Analysis Gene expression/network Analysis Biochemical and Genetic Network Prediction Molecular Interaction Prediction Biochemical Pathways/Dynamics Other Chemical Species Multi-organism function: e. g. Infectious disease Cell Behavior & Engineering Tissue Mechanics Cytomechanical Analysis Cancer Dynamics Physical properties Protein 3° Struct Macromolecular Dynamics Chromatin Structure Protein Function ID Protein Sequence ID RNA Function ID Homology Modeling RNA 3° Struct m. RNA Splicing RNA 2° Struct Proteins/RNAs m. RNA Regulation Genes/Regulatory Sequence Genome Sequence Finding Parts ORF Identification DNA Regulatory ID Assembled Genomes RNA Gene ID Polymorphisms

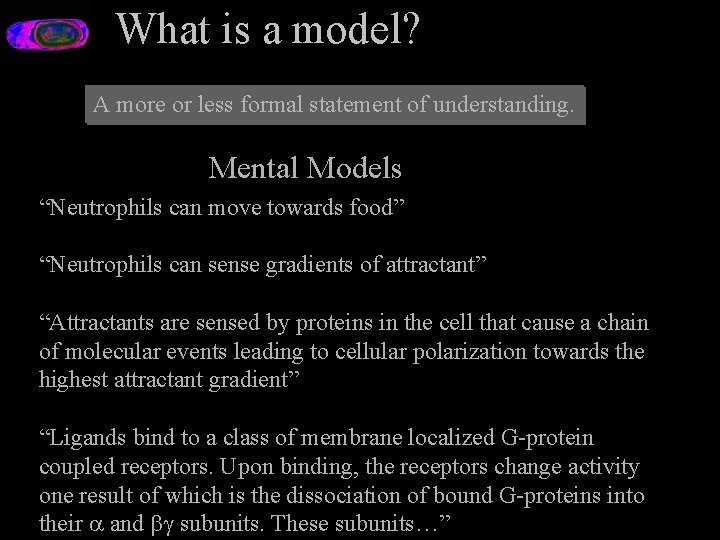
What is a model? A more or less formal statement of understanding. Mental Models “Neutrophils can move towards food” “Neutrophils can sense gradients of attractant” “Attractants are sensed by proteins in the cell that cause a chain of molecular events leading to cellular polarization towards the highest attractant gradient” “Ligands bind to a class of membrane localized G-protein coupled receptors. Upon binding, the receptors change activity one result of which is the dissociation of bound G-proteins into their and subunits. These subunits…”

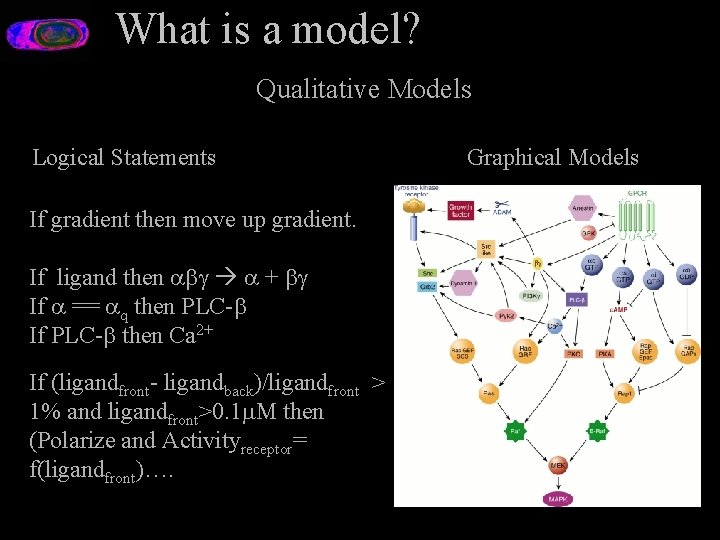
What is a model? Qualitative Models Logical Statements If gradient then move up gradient. If ligand then + If == q then PLC- If PLC- then Ca 2+ If (ligandfront- ligandback)/ligandfront > 1% and ligandfront>0. 1 M then (Polarize and Activityreceptor= f(ligandfront)…. Graphical Models

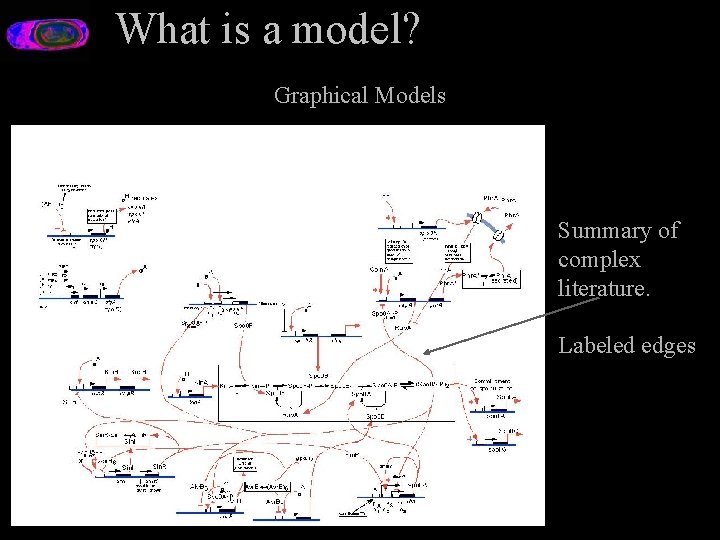
What is a model? Graphical Models Summary of complex literature. Labeled edges

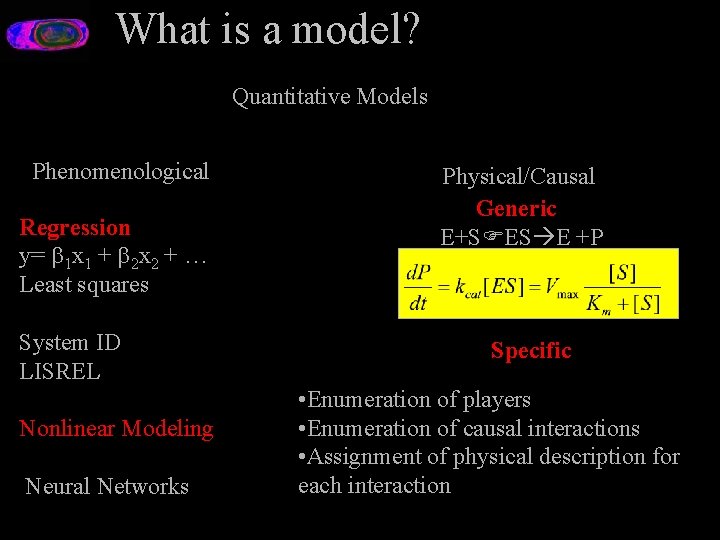
What is a model? Quantitative Models Phenomenological Regression y= 1 x 1 + 2 x 2 + … Least squares System ID LISREL Nonlinear Modeling Neural Networks Physical/Causal Generic E+S ES E +P Specific • Enumeration of players • Enumeration of causal interactions • Assignment of physical description for each interaction

Why Use More Abstract Models? 1) Not enough data 2) Model complexity too high 3) Computational Efficiency 4) Understanding

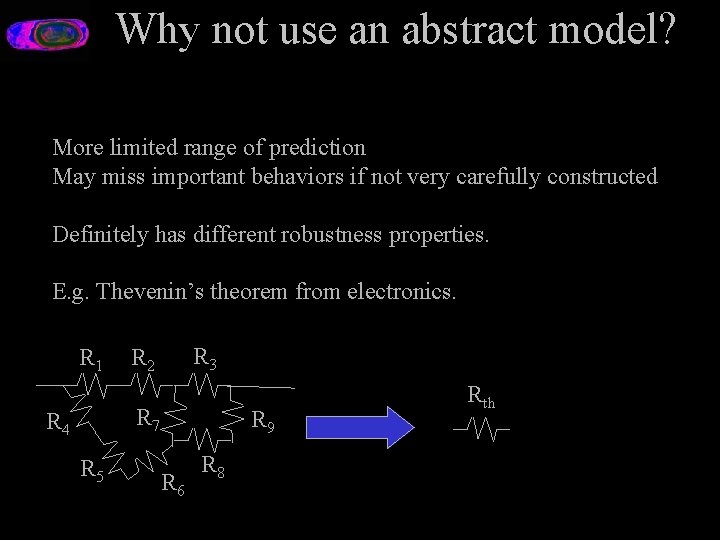
Why not use an abstract model? More limited range of prediction May miss important behaviors if not very carefully constructed Definitely has different robustness properties. E. g. Thevenin’s theorem from electronics. R 1 R 3 R 2 R 7 R 4 R 5 R 9 R 6 R 8 Rth

Why use models? Augment Intuition Systematize Data Formally Check Consistency of Knowledge Test and Create Formal Hypotheses Follow the implications of a theory Virtual Experiments / Prototyping Quantity of data, complexity of relations, implications of physics

Quantity

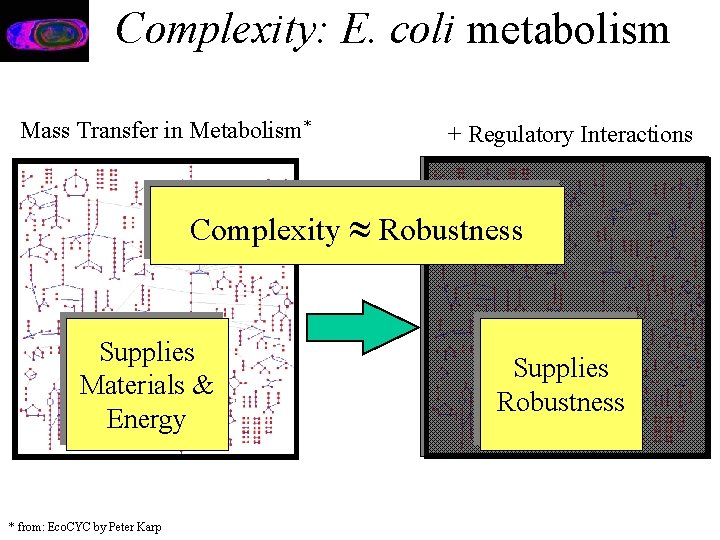
Complexity: E. coli metabolism Mass Transfer in Metabolism* + Regulatory Interactions Complexity Robustness Supplies Materials & Energy * from: Eco. CYC by Peter Karp Supplies Robustness

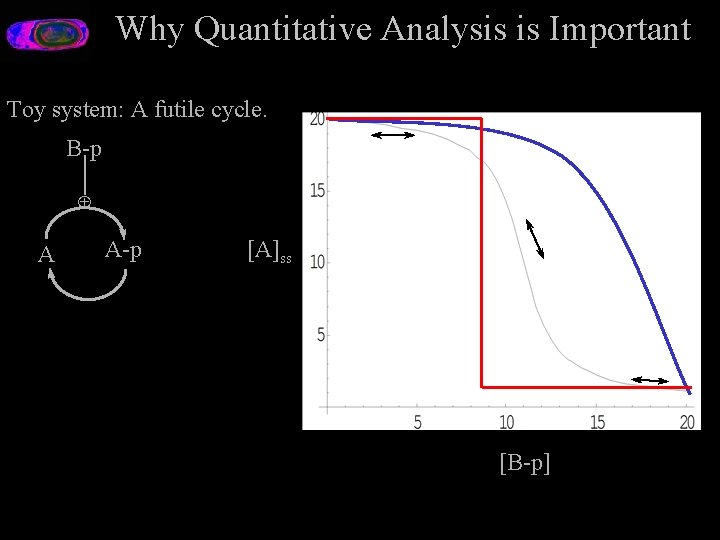
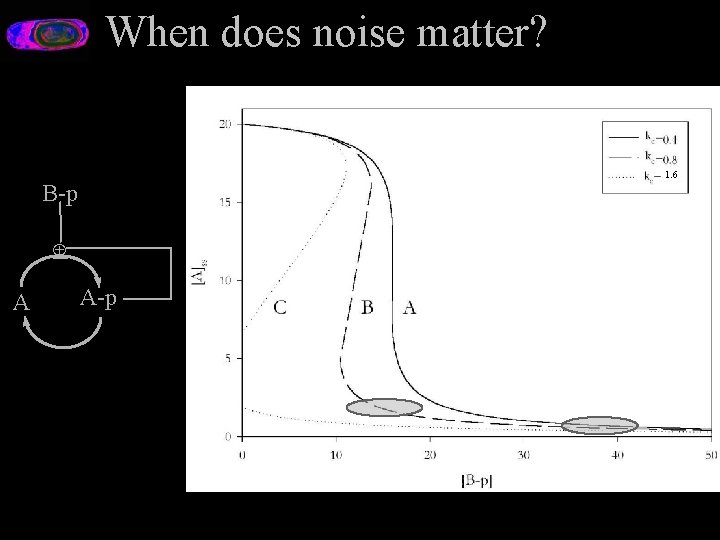
Why Quantitative Analysis is Important Toy system: A futile cycle. B-p Å A A-p [A]ss [B-p]

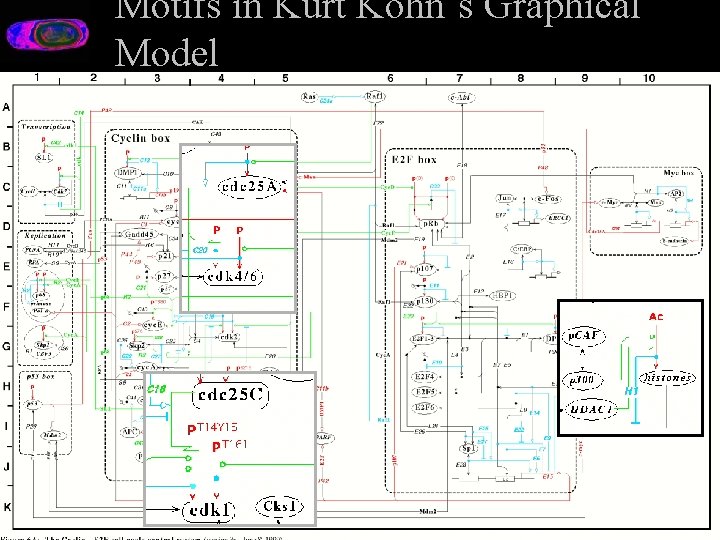
Motifs in Kurt Kohn’s Graphical Model

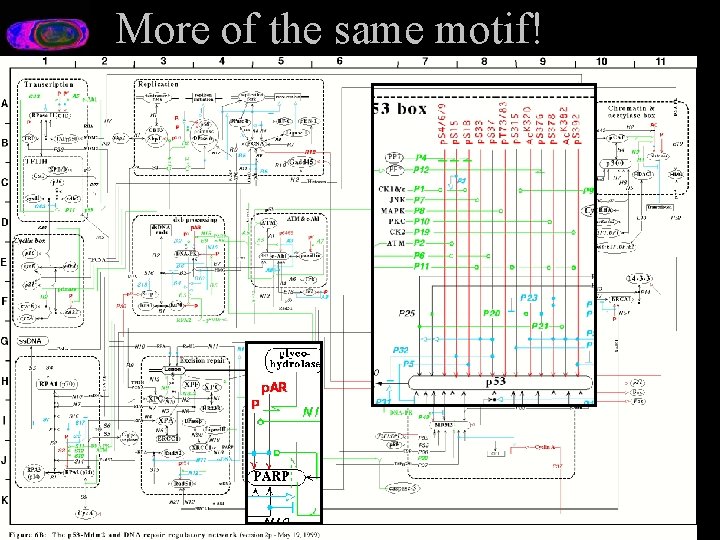
More of the same motif!
![Why is Quantitative Analysis Important Toy system A futile cyle Bp Ass Å Ap Why is Quantitative Analysis Important? Toy system: A futile cyle. B-p [A]ss Å A-p](https://slidetodoc.com/presentation_image_h2/fbf4d238e9b66bb1a07f7fe2796862cc/image-15.jpg)
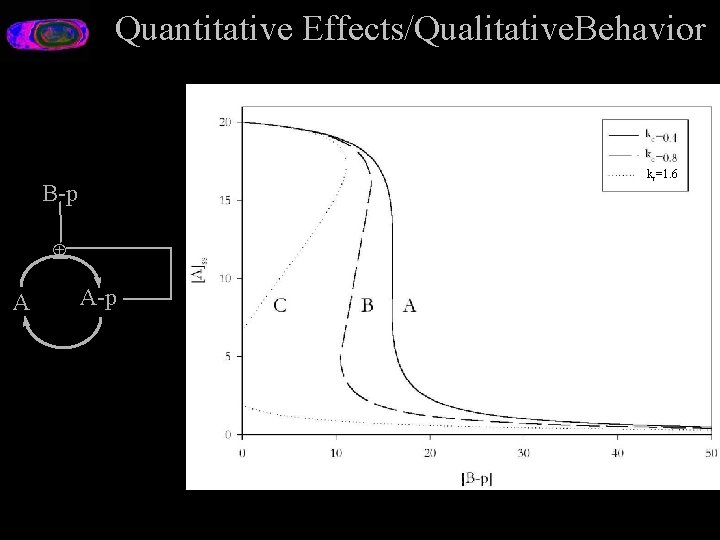
Why is Quantitative Analysis Important? Toy system: A futile cyle. B-p [A]ss Å A-p A [B-p] B-p Å A A-p ? E. g. Focal Adhesion Kinase Alternative Splice

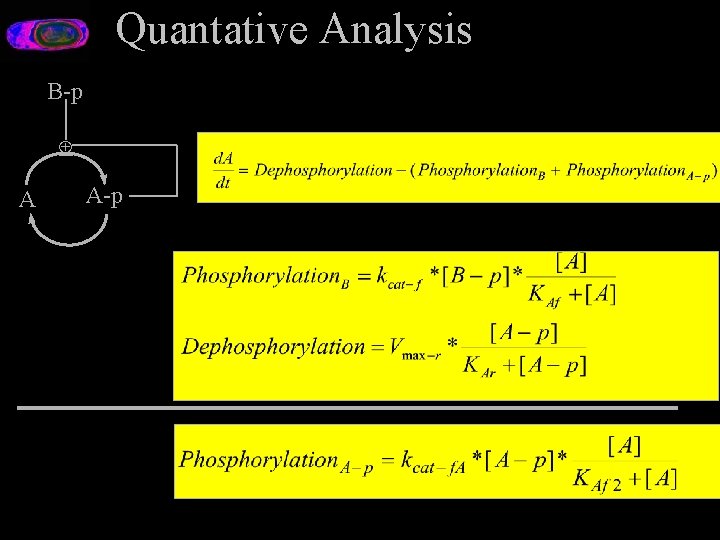
Quantative Analysis B-p Å A A-p

Quantitative Effects/Qualitative. Behavior kc=1. 6 B-p Å A A-p

Rigorous Consideration of Physics In order to create a formal description of your knowledge of the system you are forced to contend with your substrate in depth.

Some Stochastic Phenomena • Lineage commitment in human hemopoiesis • Random, bimodal eukaryotic gene transcription in – Activated T cells – Steroid hormone activation of mouse mammary tumor virus – HIV-1 virus • Clonal variation in: – Bacterial chemotactic responses – Cell cycle timing • E. coli type-1 pili expression – Enhances virulence • Changing cell surface protein expression – For immune response avoidance • Bacteriophage l lysis/lysogeny decision

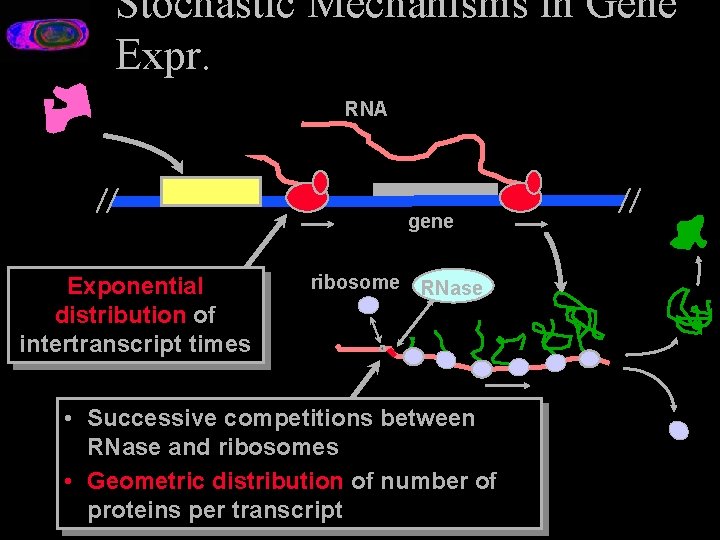
Where noise comes from • Random environmental influences • Mutations • Asymmetric partitioning at cell division • Stochastic mechanisms in gene expression – Stochastic timing of gene expression – Random variation in time for signal propagation – Random variation total protein production

Stochastic Mechanisms in Gene Expr. RNA gene Exponential distribution of intertranscript times ribosome RNase • Successive competitions between RNase and ribosomes • Geometric distribution of number of proteins per transcript

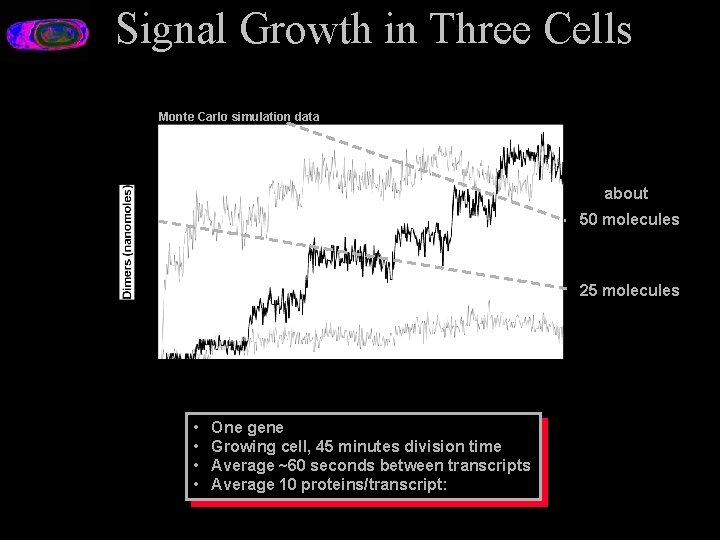
Why a Stochastic Simulation • Genes present in small numbers in the cell • Random mechanisms of transcription and translation – Random time intervals between transcripts « Average intervals can be many seconds – Random number of proteins per transcript • The presence of noise makes qualitative reasoning about regulation in a genetic network difficult • Classical coupled kinetic equation models assume continuous and deterministic processes

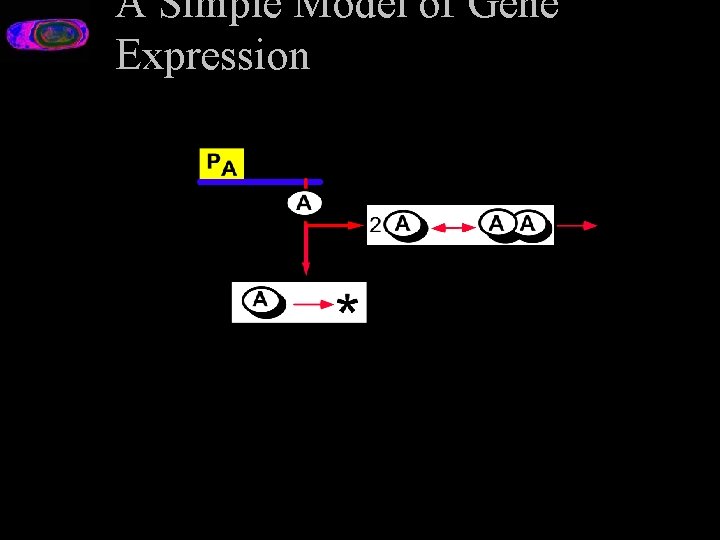
A Simple Model of Gene Expression

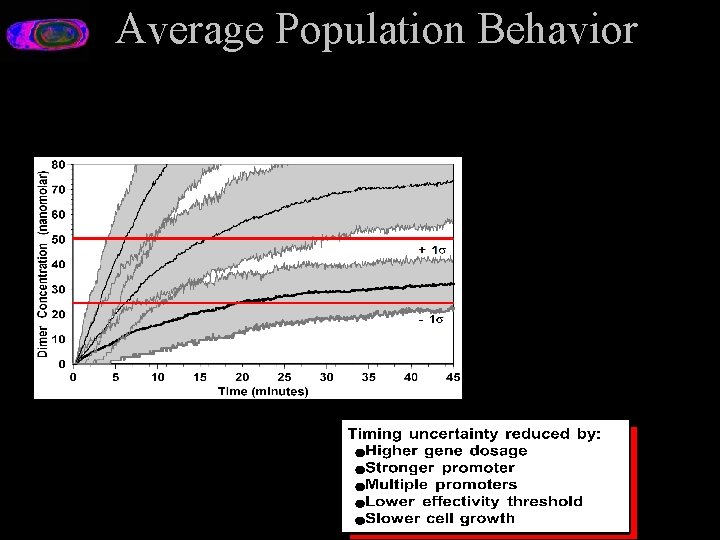
Average Population Behavior

Signal Growth in Three Cells Monte Carlo simulation data about 50 molecules 25 molecules • • One gene Growing cell, 45 minutes division time Average ~60 seconds between transcripts Average 10 proteins/transcript:

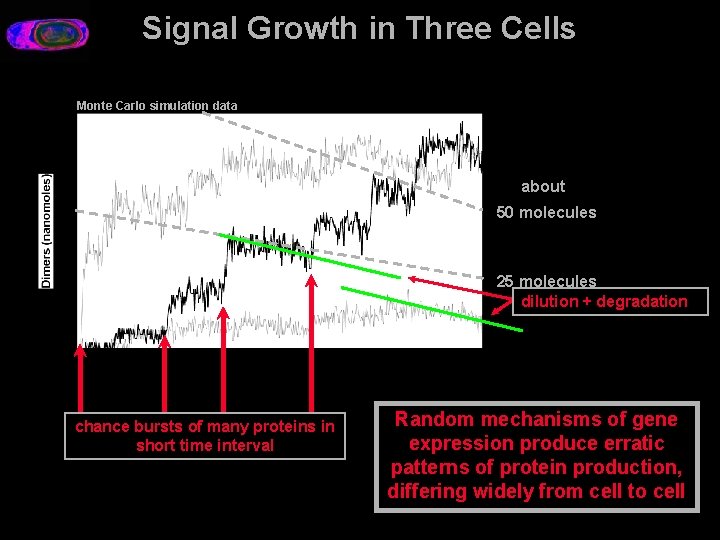
Signal Growth in Three Cells Monte Carlo simulation data about 50 molecules 25 molecules dilution + degradation chance bursts of many proteins in short time interval Random mechanisms of gene expression produce erratic patterns of protein production, differing widely from cell to cell

Evidence for Stochastic Gene Expression Novick, A. & Weiner, M. (1957) Proc. Natl. Acad. Sci. USA 43, 553– 566. Mixed Population Response to Induction of lac Promoter Siegele, D. A. & Hu, J. C. (1997) Proc. Natl. Acad. Sci. USA 94, 8168– 8172 Gene expression from plasmids containing the ara. BAD promoter at subsaturating inducer concentrations represents mixed populations Zlokarnik G, Negulescu PA, Knapp TE, Mere L, Burres N, Feng L, Whitney M, Roemer K, Tsien RY, (1998) Science 279, 84 -8 Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Ko, M. S. H. (1992) Bio. Essays 14, 341 -6 Stochastic Induction of Mouse Mammary Tumor Virus Promoter

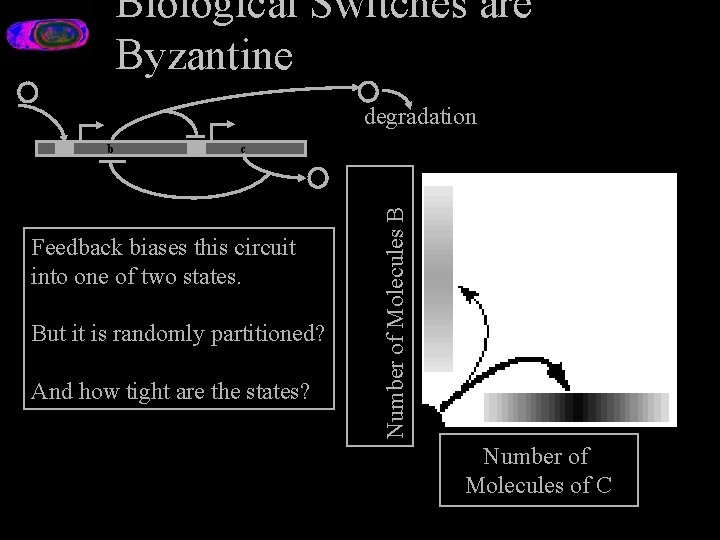
Biological Switches are Byzantine B A degradation b c Feedback biases this circuit into one of two states. But it is randomly partitioned? And how tight are the states? Number of Molecules B C Number of Molecules of C

When does noise matter? 1. 6 B-p Å A A-p

Choosing a Model Type Not so simple: When can we abstract? Noise doesn’t matter in the first case, does in the second. With such a simple model we can handcraft a solution. But for more complex systems we need an automatic way to do it. Theory is what gets us there. Maybe. One day. I hope.

Model Uniqueness and Necessity To explain data: There is rarely a sole model structure that is explanatory. Within a model structure, parameter sets are rarely unique. But they may be experimentally distinguishable! Certain data imply that certain “abstract” structures MUST be in the model. John will get to that!

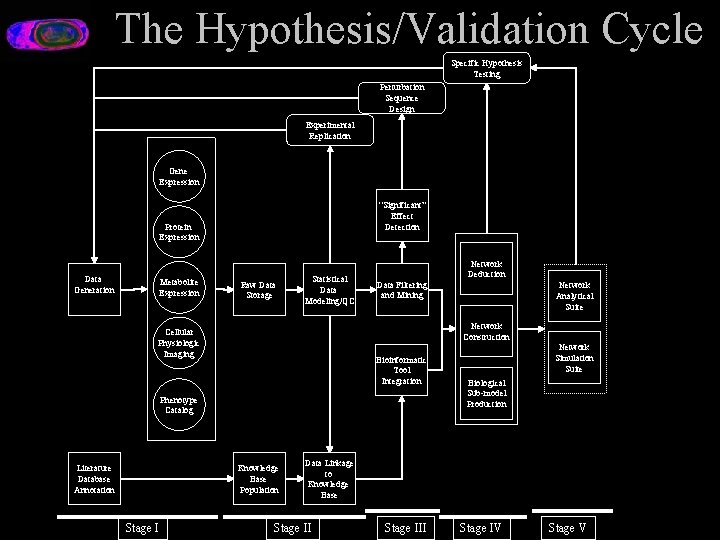
The Hypothesis/Validation Cycle Specific Hypothesis Testing Perturbation Sequence Design Experimental Replication Gene Expression “Significant” Effect Detection Protein Expression Data Generation Metabolite Expression Raw Data Storage Statistical Data Modeling/QC Network Deduction Data Filtering and Mining Network Construction Cellular Physiologic Imaging Bioinformatic Tool Integration Phenotype Catalog Literature Database Annotation Knowledge Base Population Stage I Network Analytical Suite Network Simulation Suite Biological Sub-model Production Data Linkage to Knowledge Base Stage III Stage IV Stage V

Longer Term Goals Module and Motifs Rigorous Description of Control and Sensitivity Design of control protocols: e. g. multi-Pharmaceutical control Design and implement new function in cells A theory for cellular engineering Predict, Control and Design

Prospectus What can models tell us? The big picture! How does one build analyze a physical model? Theory for models and models for Theory What is the relationship between data and modeling?