Introduction to metabolism Metabolism Anabolism Catabolism Metabolism is

Introduction to metabolism
Metabolism Anabolism Catabolism

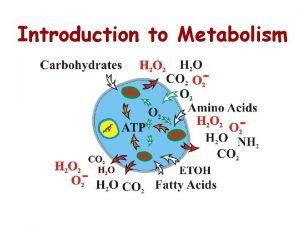
ØMetabolism is the set of chemical reactions that happen in living organisms to maintain life. ØThese processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. ØMetabolism is usually divided into two categories. ØCatabolism breaks down organic matter, for example to harvest energy in cellular respiration. ØAnabolism uses energy to construct components of cells such as proteins and nucleic acids
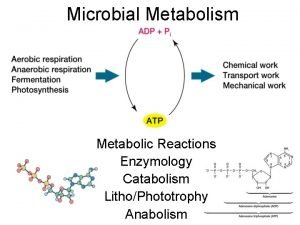
ØThe chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. ØEnzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy and will not occur by themselves, by coupling them to spontaneous reactions that release energy. ØAs enzymes act as catalysts they allow these reactions to proceed quickly and efficiently. ØEnzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or signals from other cells.

Essential macromolecules ØMost of the structures that make up animals, plants and microbes are made from three basic classes of molecule: amino acids, carbohydrates and lipids (often called fats). ØAs these molecules are vital for life, metabolic reactions focus on making these molecules during the construction of cells and tissues, or breaking them down and using them as a source of energy, in the digestion and use of food. ØMany important biochemicals can be joined together to make polymers such as DNA and proteins. ØThese macromolecules are essential.
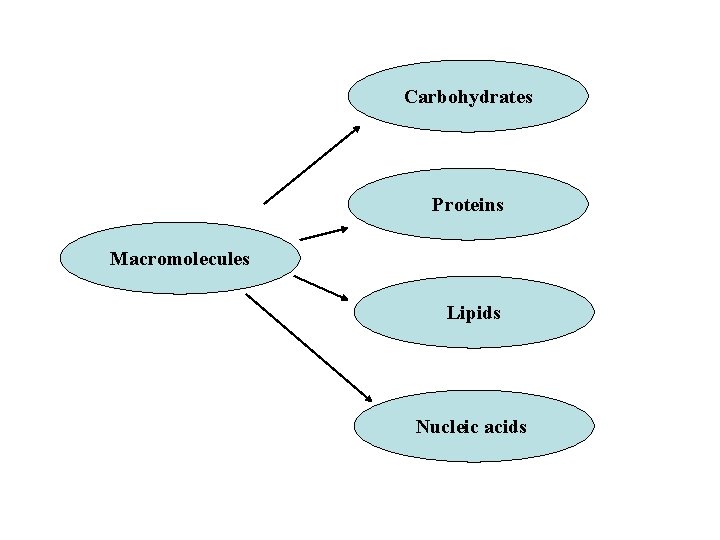
Carbohydrates Proteins Macromolecules Lipids Nucleic acids
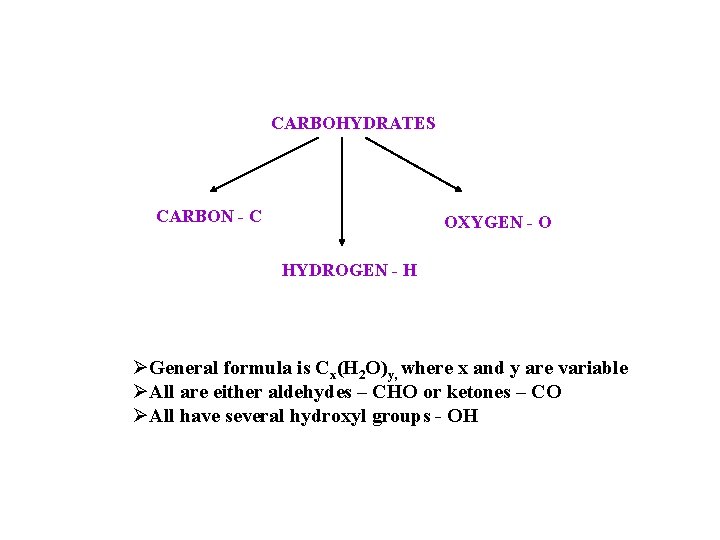
CARBOHYDRATES CARBON - C OXYGEN - O HYDROGEN - H ØGeneral formula is Cx(H 2 O)y, where x and y are variable ØAll are either aldehydes – CHO or ketones – CO ØAll have several hydroxyl groups - OH
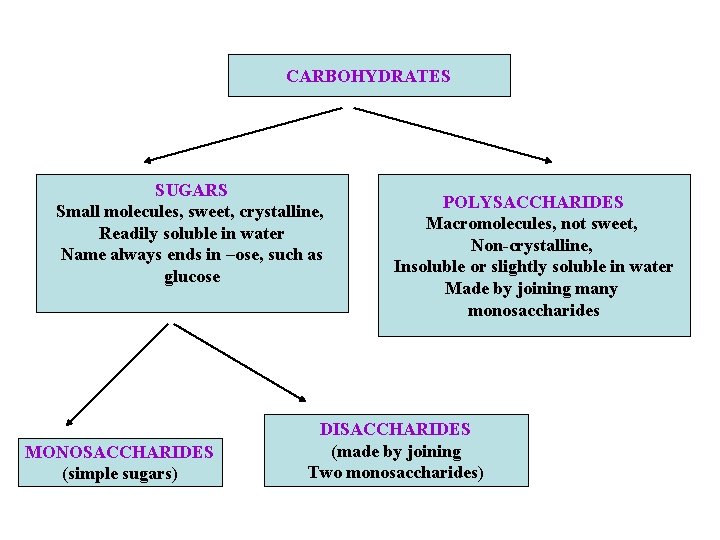
CARBOHYDRATES SUGARS Small molecules, sweet, crystalline, Readily soluble in water Name always ends in –ose, such as glucose MONOSACCHARIDES (simple sugars) POLYSACCHARIDES Macromolecules, not sweet, Non-crystalline, Insoluble or slightly soluble in water Made by joining many monosaccharides DISACCHARIDES (made by joining Two monosaccharides)
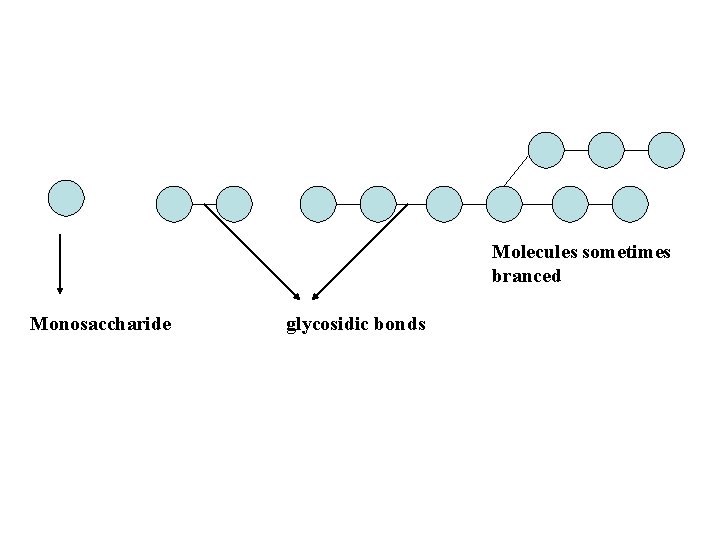
Molecules sometimes branced Monosaccharide glycosidic bonds
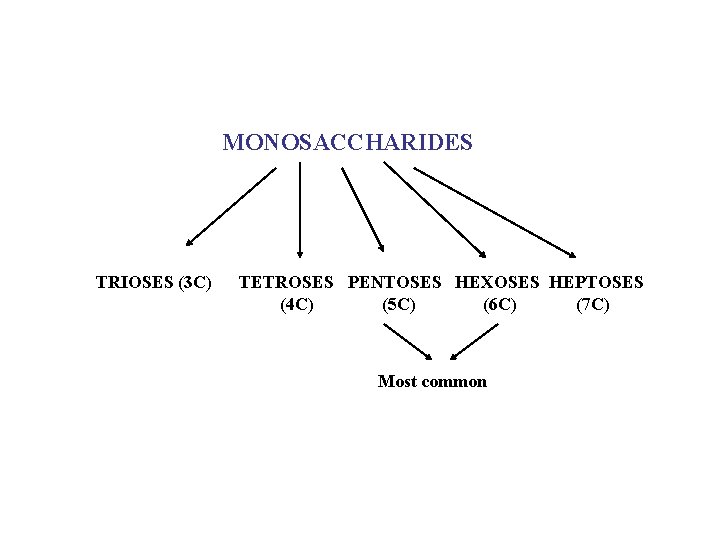
MONOSACCHARIDES TRIOSES (3 C) TETROSES PENTOSES HEXOSES HEPTOSES (4 C) (5 C) (6 C) (7 C) Most common

LIPIDS v. Water insoluble organic substances v. Can be extracted from cells by organic solvents like ether v. Formed by condensation between fatty acids and alcohol
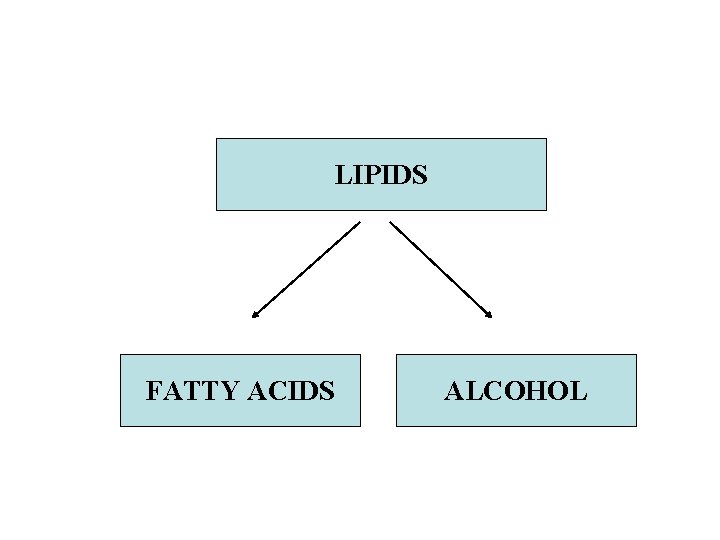
LIPIDS FATTY ACIDS ALCOHOL

FATTY ACIDS Contain acidic functional group COOH. General formula is RCOOH, where R is hydrogen or group such as CH 2. eg. Stearic acid – C 17 H 35 COOH Most fatty acids have many carbon atoms that are generally even in number, from 14 – 22.

v. Major function of lipids is to act as energy store houses. v. They have a higher calorific value than carbohydrates, because they have higher proportion of hydrogen and almost insignificant proportion of oxygen compared with carbohydrates. v. Fat in animals is stored beneath the dermis (skin) where it serves as an insulator. v. In aquatic animals such as whales, it is stored as blubber and helps in buoyancy. v. Plants store lipids as oils in seeds and fruits.
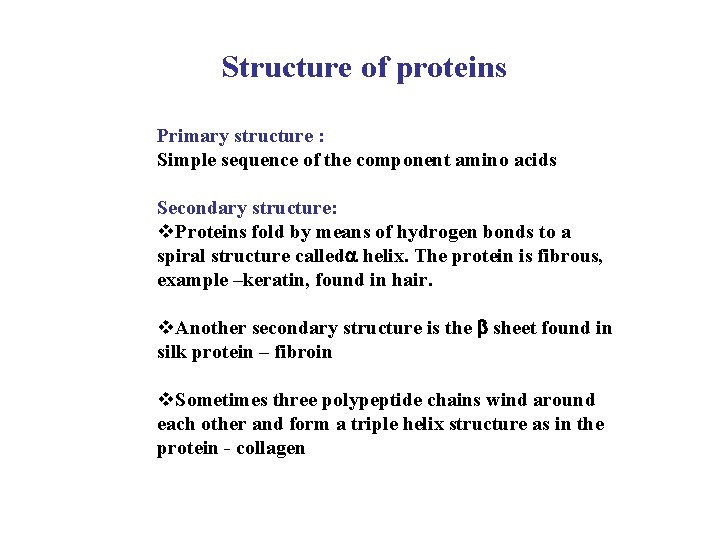
Structure of proteins Primary structure : Simple sequence of the component amino acids Secondary structure: v. Proteins fold by means of hydrogen bonds to a spiral structure called helix. The protein is fibrous, example –keratin, found in hair. v. Another secondary structure is the sheet found in silk protein – fibroin v. Sometimes three polypeptide chains wind around each other and form a triple helix structure as in the protein - collagen

Tertiary structure: Proteins fold extensively forming stable globular structures by all four types of bonds Example : myoglobin protein found in muscles. Quaternary structure: Many polypeptides coming together to form a complex molecule. Example : haemoglobin of blood, it has two alpha chains and two beta chains.
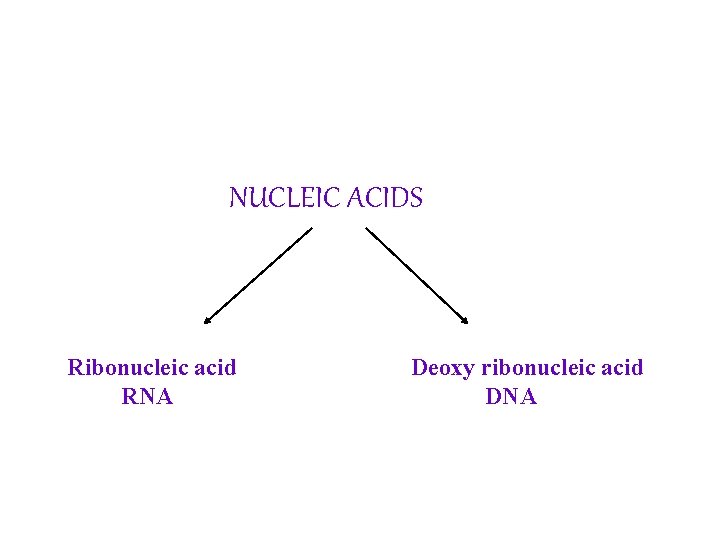
NUCLEIC ACIDS Ribonucleic acid RNA Deoxy ribonucleic acid DNA
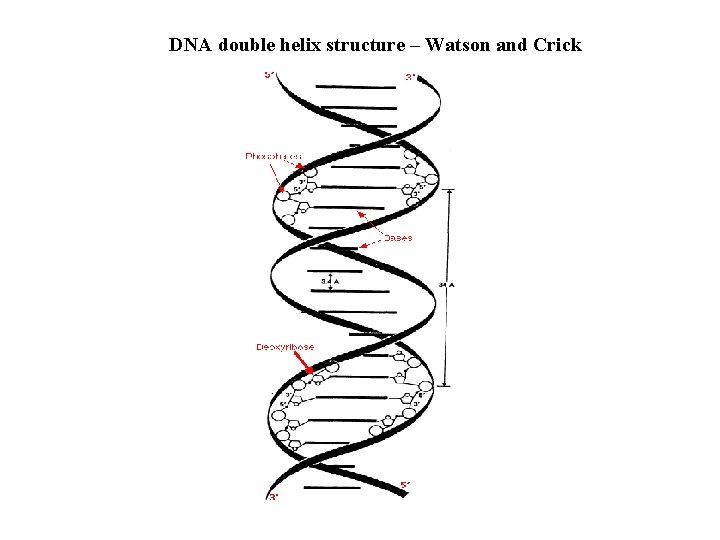
DNA double helix structure – Watson and Crick
- Slides: 18