INTRODUCTION TO MATTER MATTER HAS MASS AND VOLUME














- Slides: 18

INTRODUCTION TO MATTER

MATTER: HAS MASS AND VOLUME ANYTHING THAT HAS MASS AND OCCUPIES SPACE CHEMISTRY IS THE STUDY OF MATTER THE PROPERTIES OF DIFFERENT TYPES OF MATTER THE WAY MATTER CHANGES AND BEHAVES WHEN INFLUENCED BY OTHER MATTER AND/OR ENERGY

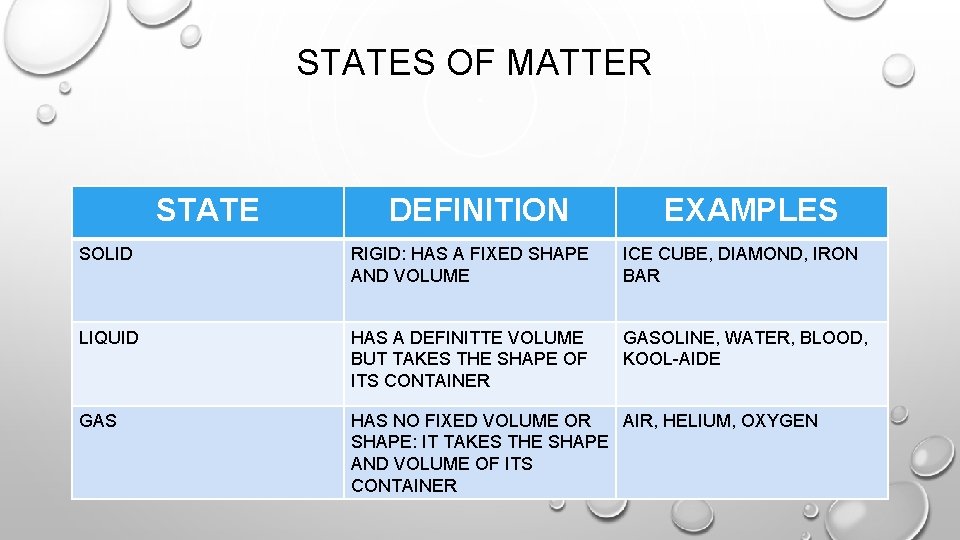
STATES OF MATTER STATE DEFINITION EXAMPLES SOLID RIGID: HAS A FIXED SHAPE AND VOLUME ICE CUBE, DIAMOND, IRON BAR LIQUID HAS A DEFINITTE VOLUME BUT TAKES THE SHAPE OF ITS CONTAINER GASOLINE, WATER, BLOOD, KOOL-AIDE GAS HAS NO FIXED VOLUME OR AIR, HELIUM, OXYGEN SHAPE: IT TAKES THE SHAPE AND VOLUME OF ITS CONTAINER

CHARACTERISTIC PROPERTIES THAT ARE TRUE FOR A TYPE OF MATTER THE SIZE THESE PROPERTIES NEVER CHANGE

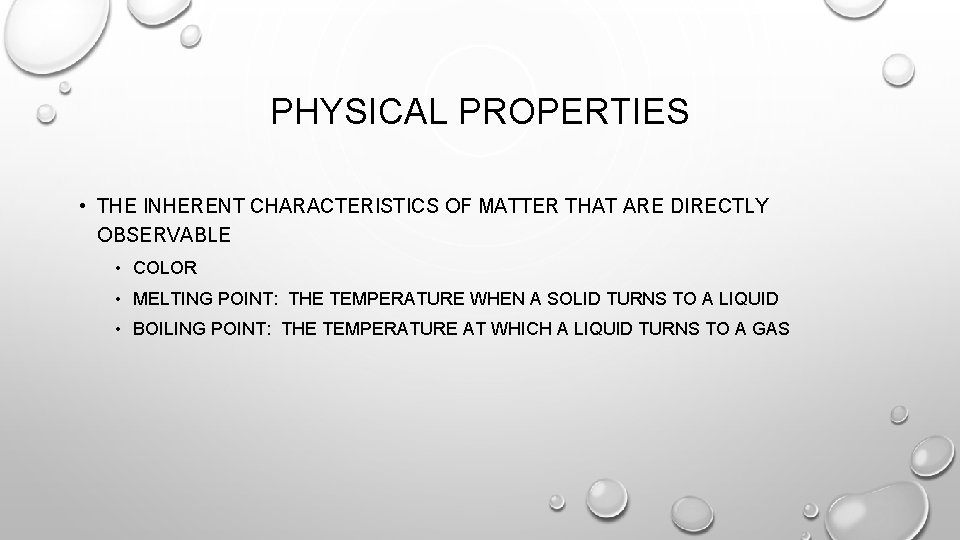
PHYSICAL PROPERTIES • THE INHERENT CHARACTERISTICS OF MATTER THAT ARE DIRECTLY OBSERVABLE • COLOR • MELTING POINT: THE TEMPERATURE WHEN A SOLID TURNS TO A LIQUID • BOILING POINT: THE TEMPERATURE AT WHICH A LIQUID TURNS TO A GAS

CHEMICAL PROPERTIES • THE CHARACTERISTICS OF MATTER THAT ALLOW IT TO FORM A NEW SUBSTANCE • COMBUSTION • ABILITY TO BURN • CORROSION • RUSTING

CLASSIFY EACH OF THE FOLLOWING AS A PHYSICAL OR CHEMICAL PROPERTY • ETHYL ALCOHOL BOILING AT 78 *C • HARDNESS OF A DIAMOND. • THE COLOR OF SOIL. • SUGAR FERMENTING TO FORM ETHYL ALCOHOL

CHANGES IN MATTER • PHYSICAL CHANGE: CHANGES TO MATTER THAT DO NOT RESULT IN A CHANGE TO THE INHERENT MAKE-UP OF THE SUBSTANCE • CHANGING STATES: BOILING, MELTING, CONDENSATION, EVAPORATION, SUBLIMATION • CHEMICAL CHANGE: CHANGES THAT INVOLVE A CHANGE IN THE FUNDAMENTAL COMPONENTS OF THE SUBSTANCE. NEW SUBSTANCE FORMED • CHEMICAL REACTIONS FORMED

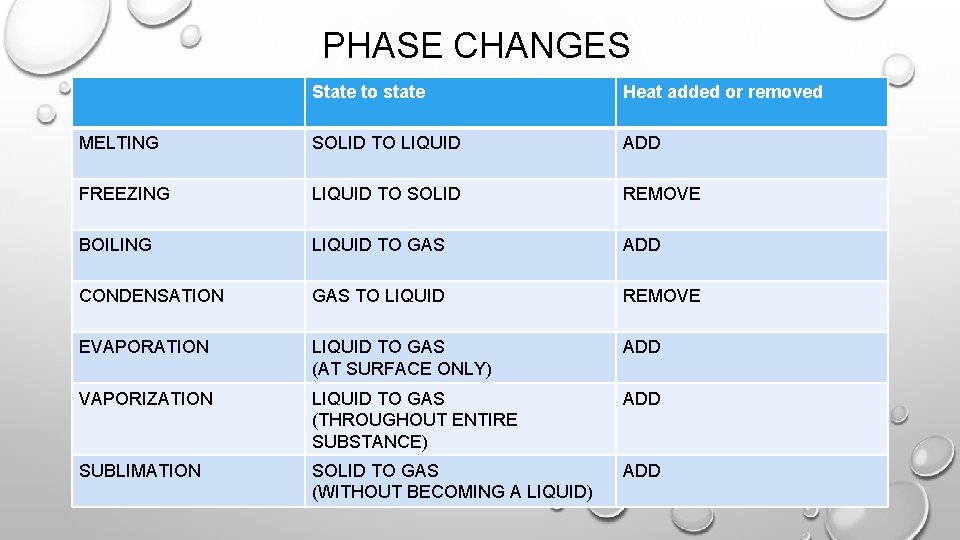
PHASE CHANGES State to state Heat added or removed MELTING SOLID TO LIQUID ADD FREEZING LIQUID TO SOLID REMOVE BOILING LIQUID TO GAS ADD CONDENSATION GAS TO LIQUID REMOVE EVAPORATION LIQUID TO GAS (AT SURFACE ONLY) ADD VAPORIZATION LIQUID TO GAS (THROUGHOUT ENTIRE SUBSTANCE) ADD SUBLIMATION SOLID TO GAS (WITHOUT BECOMING A LIQUID) ADD

CLASSIFY EACH OF THE FOLLOWING AS A PHYSICAL OR CHEMICAL CHANGE • IRON METAL MELTING • IRON COMBINING WITH OXYGEN TO FORM RUST • SUGAR FERMENTING TO FORM ETHYL ALCOHOL

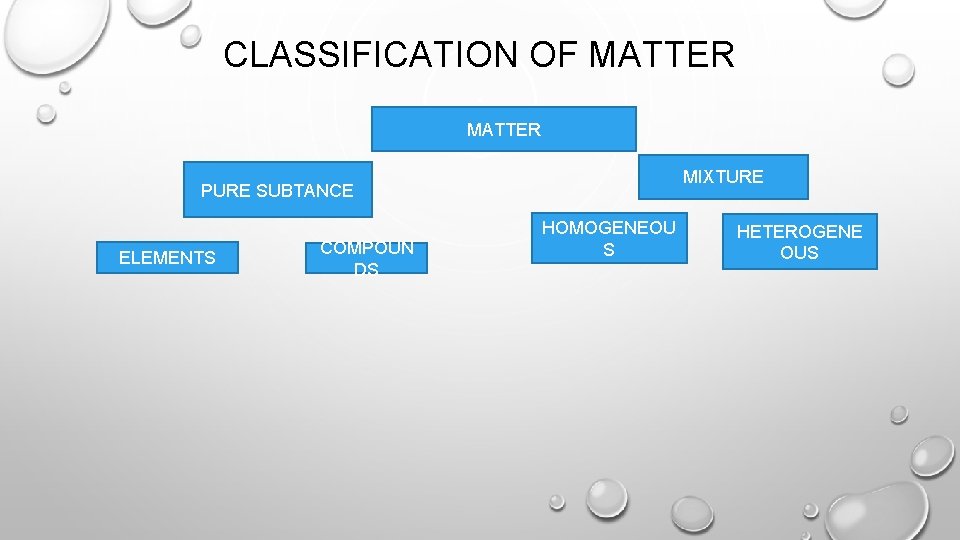
CLASSIFICATION OF MATTER MIXTURE PURE SUBTANCE ELEMENTS COMPOUN DS HOMOGENEOU S HETEROGENE OUS

HOMOGENEOUS MATTER • UNIFORM COMPOSITION THROUGHOUT THE SUBSTANCE • ALL PURE SUBSTANCES • SOLUTIONS: WELL MIXED MIXTURE • SOLVENT: THE SUBSTANCE IN WHICH SOMETHING IS DISSOLVED • SOLUTE : THE SUBSTANCE WHICH IS DISSOLVED

HETEROGENEOUS MIXTURE • NON-UNIFORM: CONTAINS REGIONS WITH DIFFERENT PROPERTIES THAN OTHER REGIONS

PURE SUBSTANCES • ALL SAMPLES HAVE THE SAME PHYSICAL AND CHEMICAL PROPERTIES • CONSTANT COMPOSITION • THEY ARE HOMOGENEOUS • ELEMENTS AND COMPOUNDS EXAMPLES

ELEMENTS AND COMPOUNDS • ELEMENTS: SUBSTANCES THAT CANNOT BE BROKEN DOWN INTO SIMPLER SUBSTANCES BY CHEMICAL REACTIONS • MOST SUBSTANCES ARE CHEMICAL COMBINATIONS OF ELEMENTS. THESE COMBINATIONS ARE CALLED COMPOUNDS. • COMPOUNDS ARE MADE OF ELEMENTS • COMPOUNDS CAN BE BROKEN DOWN INTO ELEMENTS

MIXTURES • DIFFERENT SAMPLES MAY SHOW DIFFERENT PROPERTIES • VARIABLE COMPOSITION • HOMOGENEOUS OR HETEROGENEOUS • SEPARATE INTO COMPONENTS BASED ON PHYSICAL PROPERTIES • ALL MIXTURES ARE MADE OF PURE SUBSTANCES

SOLUTIONS • A SOLUTION IS A HOMOGENEOUS MIXTURE • PHASE CAN BE GASEOUS, LIQUID, OR SOLID

IDENTIFY EACH OF THE FOLLOWING AS A PURE SUBSTANCE, HOMOGENEOUS OR HETEROGENOUS MIXTURE • GASOLINE • A STREAM WITH GRAVEL ON THE BOTTOM • COPPER METAL