Introduction to mathematical modeling of physical and chemical


![Dissociation in Liquid Phase [SO 2]in =[SO 2]+ [SO 32 - ] + [HSO Dissociation in Liquid Phase [SO 2]in =[SO 2]+ [SO 32 - ] + [HSO](https://slidetodoc.com/presentation_image/0bcbd5a65fabd0b321e74f787d65f08f/image-16.jpg)






- Slides: 39

Introduction to mathematical modeling of physical and chemical processes in the EBFGT Drd. MSc Valentina Gogulancea Prof. dr. eng. Ioan Calinescu Prof. dr. eng. Vasile Lavric 1

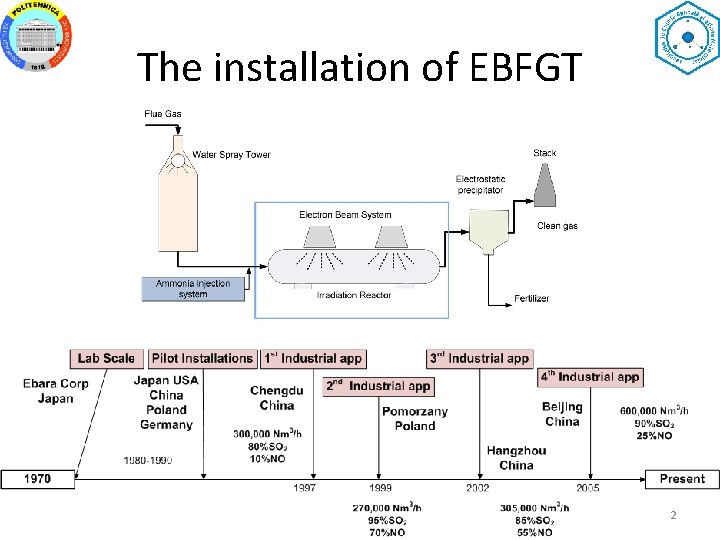
The installation of EBFGT 2

Historical Developments 1. Nishimura, K. & Suzuki, N. 1981. Radiation Treatment of Exhaust Gases - Analysis of NO Oxidation and Decomposition in Dry and Moist NO-O 2 -N 2 Mixtures by Computer Simulation. Journal of Nuclear Science and Technology, 18, 878 -886. 2. Mätzing, H. 1991. Chemical Kinetics of Flue Gas Cleaning by Irradiation with Electrons. Advances in Chemical Physics, volume 80, Ed I Prigogine & S. Rice. John Wiley & Sons, Inc. 3. Paur, H. R. & Matzing, H. 1993. Electron-Beam-Induced Purification of Dilute Off Gases from Industrial-Processes and Automobile Tunnels. Radiation Physics and Chemistry, 42, 719 -722. 4. Matzing, H. , Namba, H. & Tokunaga, O. 1993. Kinetics of SO 2 Removal from Flue-Gas by Electron-Beam Technique. Radiation Physics and Chemistry, 42, 673 -677. 5. Penetrante, B. M. 1996. Flue Gas Dry Scrubbing Using Pulsed Electron Beams. Second International Symposium on Environmental Applications of Advanced oxidation Technologies. San Francisco, CA. 6. Penetrante, B. M. 1997. Fundamental limits on NOx reduction by plasma. Technical report – SAE Publications Group 7. Penetrante, B. M. 1997. Kinetic Analysis of Non-Thermal Plasmas Used for Pollution Control, Japanese Journal of Applied Physics, 36, pp 5007 -5017 8. Gerasimov, G. Y. , Gerasimova, T. S. , Makarov, V. N. & Fadeev, S. A. 1996. Homogeneous and heterogeneous radiation induced NO and SO 2 removal from power plants flue gases - Modeling study. Radiation Physics and Chemistry, 48, 763 -769. 9. Li, R. N. , Yan, K. P. , Miao, J. S. & Wu, X. L. 1998. Heterogeneous reactions in non-thermal plasma flue gas desulfurization. Chemical Engineering Science, 53, 1529 -1540. 10. Zhang, J. , Sun, J. , Gong, Y. , Wang, D. , Ma, T. & Liu, Y. 2009. A scheme for solving strongly coupled chemical reaction equations appearing in the removal of SO 2 and NOx from flue gases. Vacuum, 83, 133 -137. 11. Schmitt, K. L. , Murray, D. M. & Dibble, T. S. 2009. Towards a Consistent Chemical Kinetic Model of Electron Beam Irradiation of Humid Air. Plasma Chemistry and Plasma Processing, 29, 347 -362. 12. Schmitt, K. L. & Dibble, T. S. 2011. Understanding OH Yields in Electron Beam Irradiation of Humid N(2). Plasma Chemistry and Plasma Processing, 31, 41 -50. 3

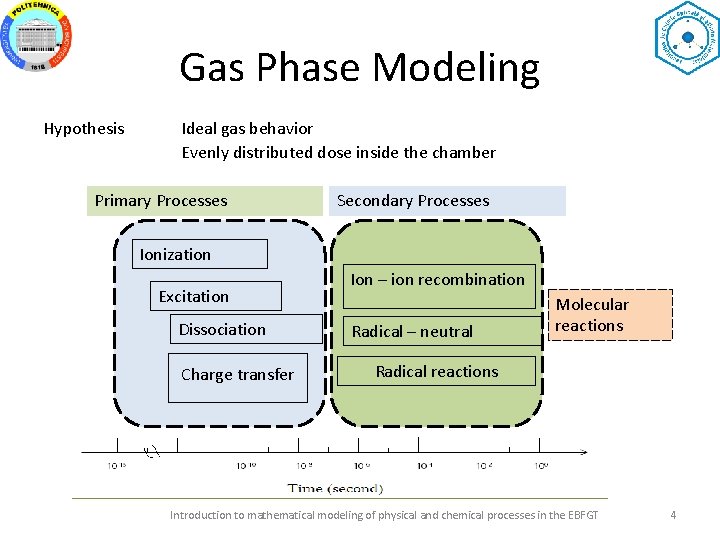
Gas Phase Modeling Hypothesis Ideal gas behavior Evenly distributed dose inside the chamber Primary Processes Secondary Processes Ionization Excitation Dissociation Charge transfer Ion – ion recombination Radical – neutral Molecular reactions Radical reactions Introduction to mathematical modeling of physical and chemical processes in the EBFGT 4

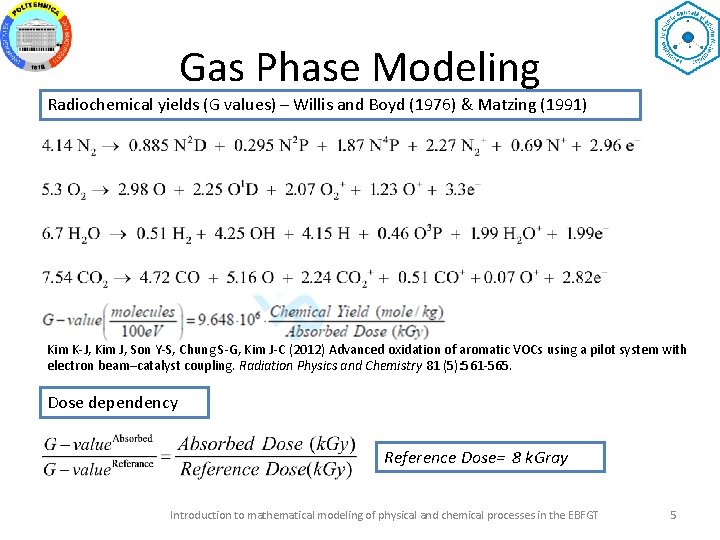
Gas Phase Modeling Radiochemical yields (G values) – Willis and Boyd (1976) & Matzing (1991) Kim K-J, Kim J, Son Y-S, Chung S-G, Kim J-C (2012) Advanced oxidation of aromatic VOCs using a pilot system with electron beam–catalyst coupling. Radiation Physics and Chemistry 81 (5): 561 -565. Dose dependency Reference Dose= 8 k. Gray Introduction to mathematical modeling of physical and chemical processes in the EBFGT 5

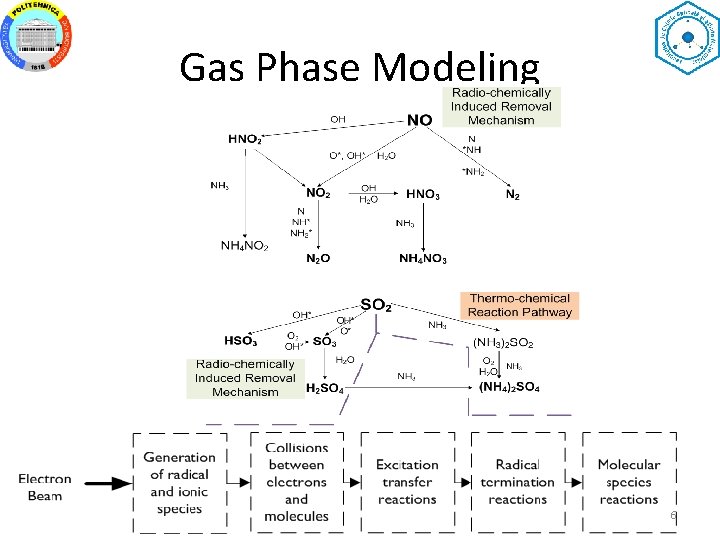
Gas Phase Modeling 6

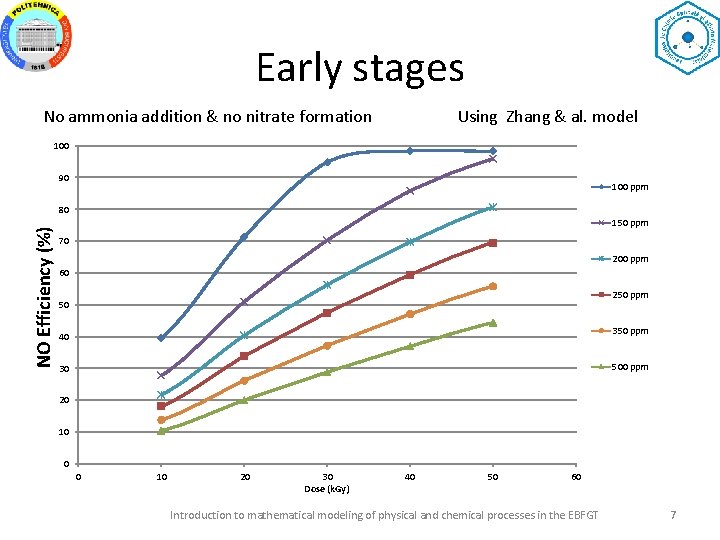
Early stages No ammonia addition & no nitrate formation Using Zhang & al. model 100 90 100 ppm NO Efficiency (%) 80 150 ppm 70 200 ppm 60 250 ppm 50 350 ppm 40 500 ppm 30 20 10 0 0 10 20 30 Dose (k. Gy) 40 50 60 Introduction to mathematical modeling of physical and chemical processes in the EBFGT 7

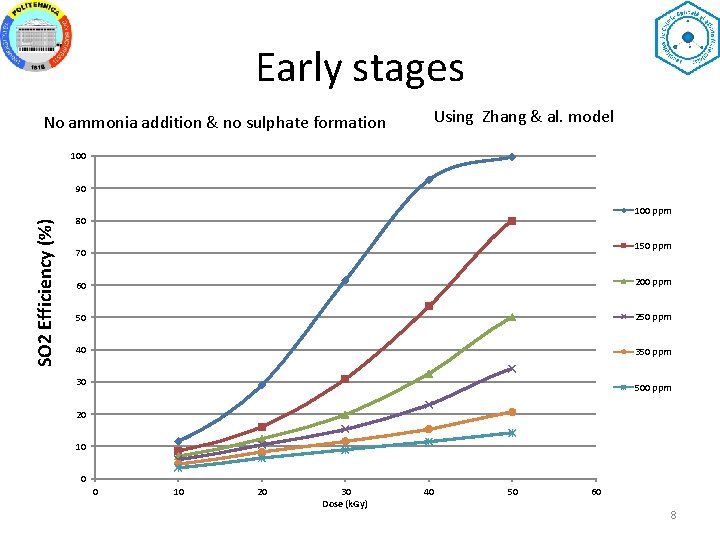
Early stages No ammonia addition & no sulphate formation Using Zhang & al. model 100 SO 2 Efficiency (%) 90 100 ppm 80 150 ppm 70 60 200 ppm 50 250 ppm 40 350 ppm 30 500 ppm 20 10 0 0 10 20 30 Dose (k. Gy) 40 50 60 8

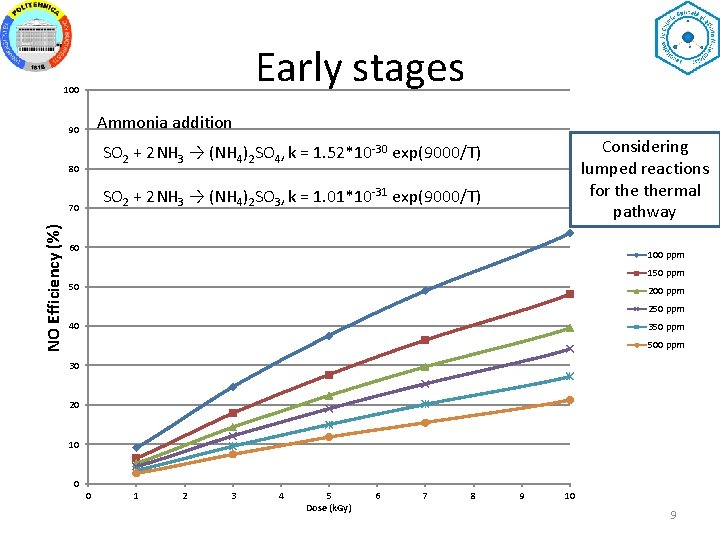
Early stages 100 Ammonia addition 90 80 SO 2 + 2 NH 3 → (NH 4)2 SO 3, k = 1. 01*10 -31 exp(9000/T) 70 NO Efficiency (%) Considering lumped reactions for thermal pathway SO 2 + 2 NH 3 → (NH 4)2 SO 4, k = 1. 52*10 -30 exp(9000/T) 60 100 ppm 150 ppm 50 200 ppm 250 ppm 40 350 ppm 500 ppm 30 20 10 0 0 1 2 3 4 5 Dose (k. Gy) 6 7 8 9 10 9

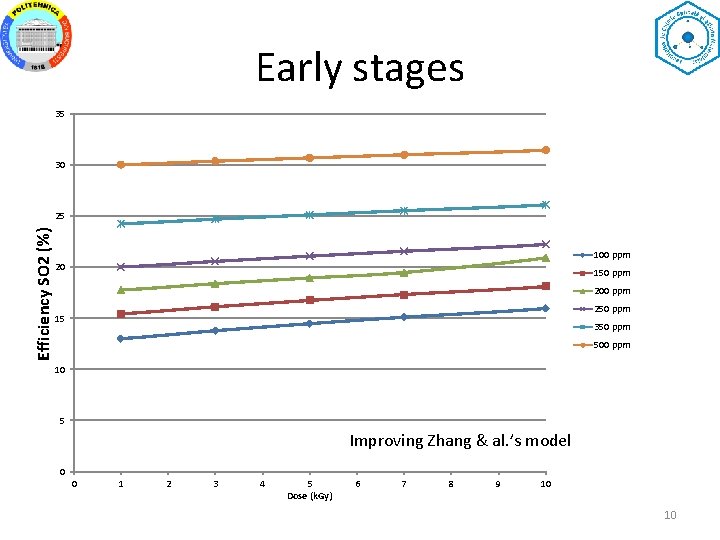
Early stages 35 30 Efficiency SO 2 (%) 25 100 ppm 20 150 ppm 200 ppm 250 ppm 15 350 ppm 500 ppm 10 5 Improving Zhang & al. ’s model 0 0 1 2 3 4 5 Dose (k. Gy) 6 7 8 9 10 10

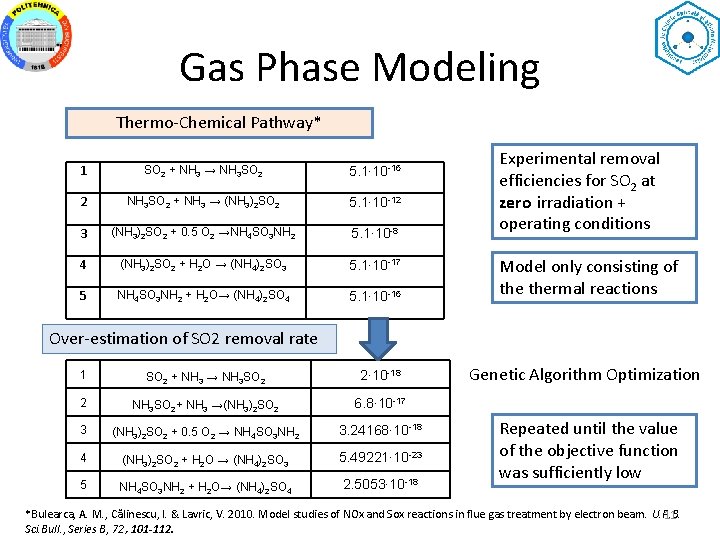
Gas Phase Modeling Thermo-Chemical Pathway* 1 SO 2 + NH 3 → NH 3 SO 2 5. 1∙ 10 -16 2 NH 3 SO 2 + NH 3 → (NH 3)2 SO 2 5. 1∙ 10 -12 3 (NH 3)2 SO 2 + 0. 5 O 2 →NH 4 SO 3 NH 2 5. 1∙ 10 -8 4 (NH 3)2 SO 2 + H 2 O → (NH 4)2 SO 3 5. 1∙ 10 -17 5 NH 4 SO 3 NH 2 + H 2 O→ (NH 4)2 SO 4 5. 1∙ 10 -16 Experimental removal efficiencies for SO 2 at zero irradiation + operating conditions Model only consisting of thermal reactions Over-estimation of SO 2 removal rate 1 SO 2 + NH 3 → NH 3 SO 2 2∙ 10 -18 2 NH 3 SO 2+ NH 3 →(NH 3)2 SO 2 6. 8∙ 10 -17 3 (NH 3)2 SO 2 + 0. 5 O 2 → NH 4 SO 3 NH 2 3. 24168∙ 10 -18 4 (NH 3)2 SO 2 + H 2 O → (NH 4)2 SO 3 5. 49221∙ 10 -23 5 NH 4 SO 3 NH 2 + H 2 O→ (NH 4)2 SO 4 2. 5053∙ 10 -18 Genetic Algorithm Optimization Repeated until the value of the objective function was sufficiently low *Bulearca, A. M. , Călinescu, I. & Lavric, V. 2010. Model studies of NOx and Sox reactions in flue gas treatment by electron beam. U. P. B. 11 Sci. Bull. , Series B, 72, 101 -112.

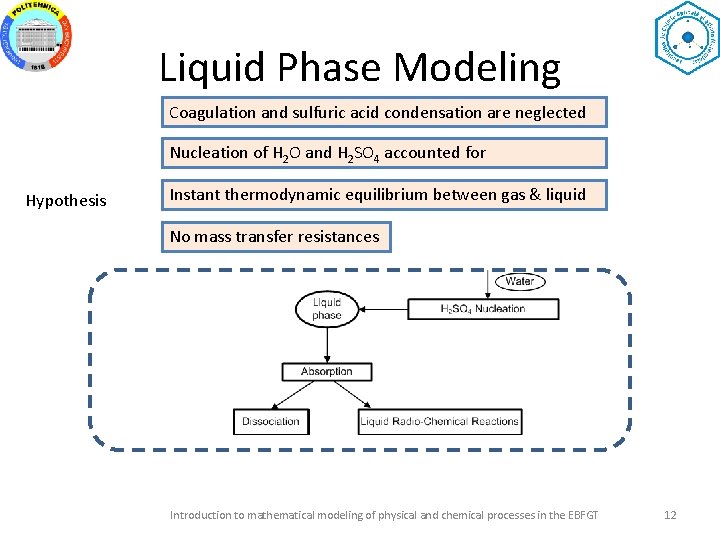
Liquid Phase Modeling Coagulation and sulfuric acid condensation are neglected Nucleation of H 2 O and H 2 SO 4 accounted for Hypothesis Instant thermodynamic equilibrium between gas & liquid No mass transfer resistances Introduction to mathematical modeling of physical and chemical processes in the EBFGT 12

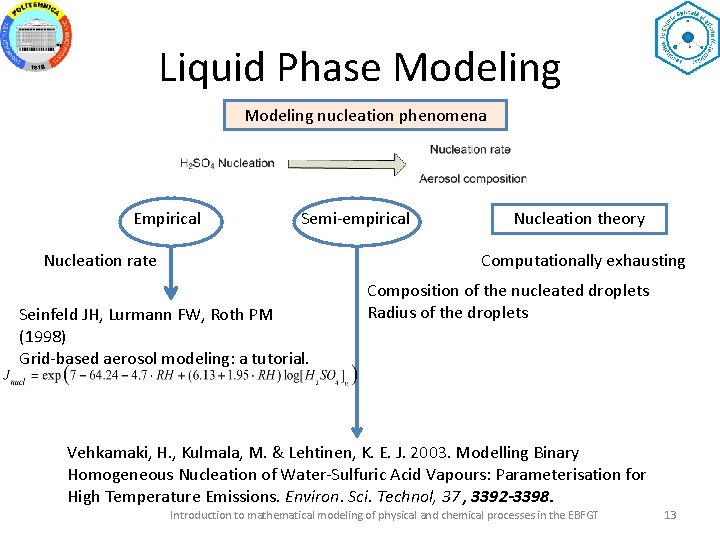
Liquid Phase Modeling nucleation phenomena Empirical Semi-empirical Nucleation rate Nucleation theory Computationally exhausting Seinfeld JH, Lurmann FW, Roth PM (1998) Grid-based aerosol modeling: a tutorial. Composition of the nucleated droplets Radius of the droplets Vehkamaki, H. , Kulmala, M. & Lehtinen, K. E. J. 2003. Modelling Binary Homogeneous Nucleation of Water-Sulfuric Acid Vapours: Parameterisation for High Temperature Emissions. Environ. Sci. Technol, 37 , 3392 -3398. Introduction to mathematical modeling of physical and chemical processes in the EBFGT 13

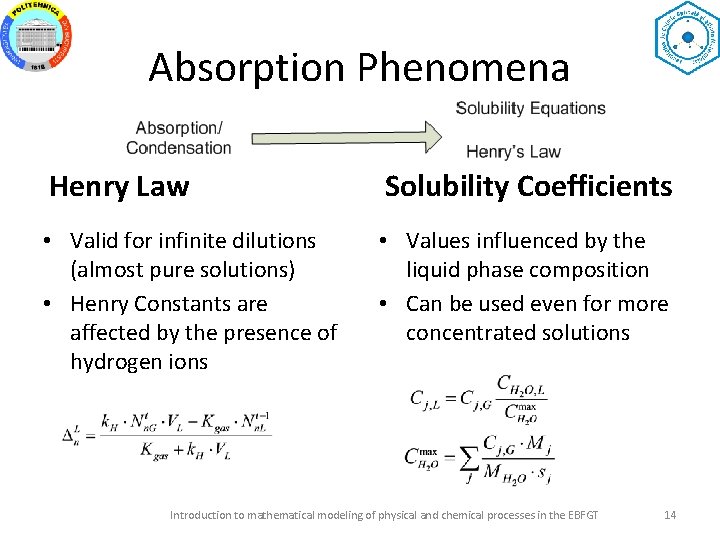
Absorption Phenomena Henry Law Solubility Coefficients • Valid for infinite dilutions (almost pure solutions) • Henry Constants are affected by the presence of hydrogen ions • Values influenced by the liquid phase composition • Can be used even for more concentrated solutions Introduction to mathematical modeling of physical and chemical processes in the EBFGT 14

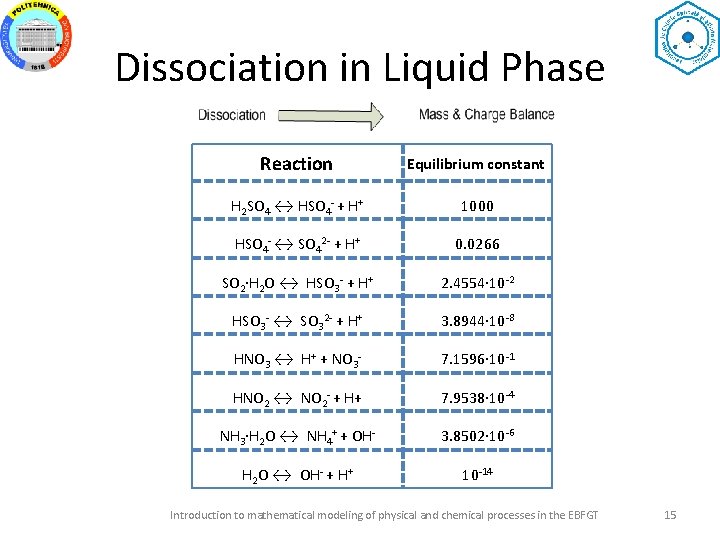
Dissociation in Liquid Phase Reaction Equilibrium constant H 2 SO 4 ↔ HSO 4 - + H+ 1000 HSO 4 - ↔ SO 42 - + H+ 0. 0266 SO 2∙H 2 O ↔ HSO 3 - + H+ 2. 4554∙ 10 -2 HSO 3 - ↔ SO 32 - + H+ 3. 8944∙ 10 -8 HNO 3 ↔ H+ + NO 3 - 7. 1596∙ 10 -1 HNO 2 ↔ NO 2 - + H+ 7. 9538∙ 10 -4 NH 3∙H 2 O ↔ NH 4+ + OH- 3. 8502∙ 10 -6 H 2 O ↔ OH- + H+ 10 -14 Introduction to mathematical modeling of physical and chemical processes in the EBFGT 15
![Dissociation in Liquid Phase SO 2in SO 2 SO 32 HSO Dissociation in Liquid Phase [SO 2]in =[SO 2]+ [SO 32 - ] + [HSO](https://slidetodoc.com/presentation_image/0bcbd5a65fabd0b321e74f787d65f08f/image-16.jpg)
Dissociation in Liquid Phase [SO 2]in =[SO 2]+ [SO 32 - ] + [HSO 3 -] [NH 3∙H 2 O]in = [NH 3∙H 2 O] + [NH 4+] -] [H 2 SO 4]in = [H 2 SO 4] + [HSO 4 + [SO 4 2 - ] Mass Balance [HNO 3]in = [HNO 3] + [NO 3 -] [HNO 2]in = [HNO 2] + [NO 2 -] [H 2 O]i = [H 2 O] + [OH -] Charge Balance [H+] + [NH 4+] = [HSO 3 -] + 2 [SO 32 -] + [HO -] + [HSO 4 -] + 2 [SO 42 -] + [NO 3 -] + [NO 2] 10 soluble species, 15 mass & charge balance equations => system of linear and non-linear algebraic equations Introduction to mathematical modeling of physical and chemical processes in the EBFGT 16

Liquid Phase Reactions Radiolysis Phenomena Dose distributed between the liquid and gas phase Introduction to mathematical modeling of physical and chemical processes in the EBFGT 17

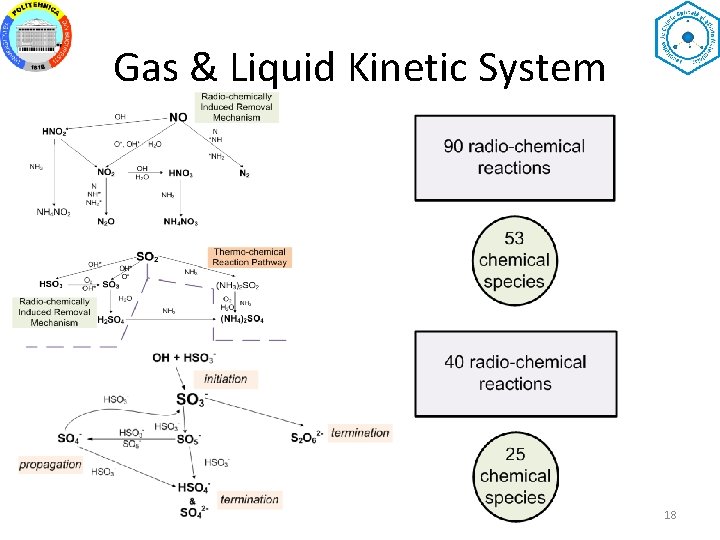
Gas & Liquid Kinetic System 18

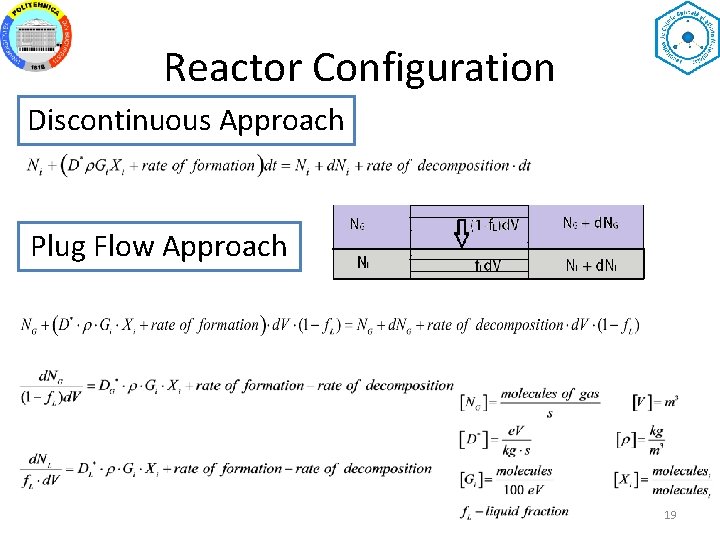
Reactor Configuration Discontinuous Approach Plug Flow Approach 19

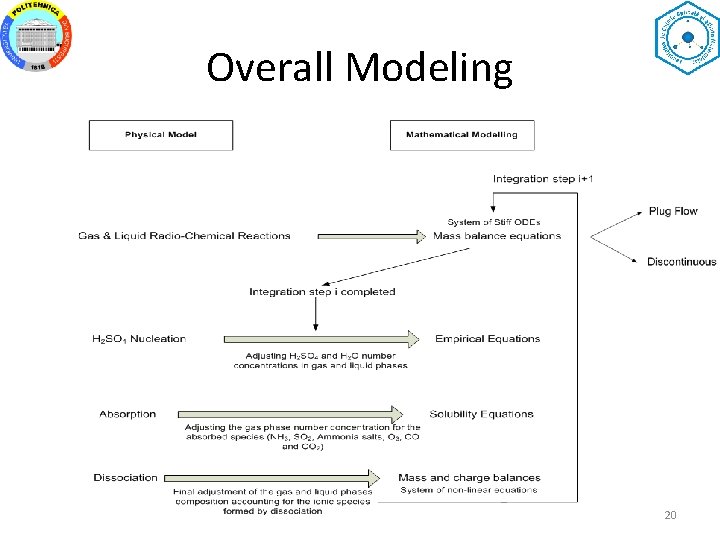
Overall Modeling 20

Model validation Chmielewski, A. G. , Tyminski, B. , Dobrowolski, A. , Iller, E. , Zimek, Z. & Licki, J. 2000. Empirical models for NOx and SO 2 removal in a double stage flue gas irradiation process. Radiation Physics and Chemistry, 57, 527 -530. Experimental conditions Experiment # Temperature (⁰C) Humidity (%) Removal efficiencies Dose (k. Gy) Residence time (s) [NO]initial (ppm) [SO 2]initial (ppm) NH 3 ratio NO (%) SO 2 (%) 1 58. 6 12. 0 10. 0 14. 43 127 383 0. 92 77. 9 93. 2 2 59. 2 10. 7 10. 0 14. 36 171 364 0. 89 72. 5 99. 2 3 60. 4 8. 6 10. 2 4. 11 161 673 0. 89 82. 1 81. 0 4 54. 9 8. 2 10. 0 13. 4 129 359 0. 88 81. 0 98. 6 5 60. 3 7. 7 10. 1 4. 05 196 467 0. 88 74. 0 74. 1 6 78. 8 6. 9 10. 1 6. 02 216 430 0. 9 74. 1 67. 7 7 55. 1 7. 9 12. 5 3. 56 157 465 0. 91 73. 9 89. 2 8 55. 8 8. 0 12. 7 3. 63 159 484 0. 88 77. 3 81. 0 9 78. 8 6. 7 10. 1 5. 99 216 421 0. 91 74. 3 10 61. 2 8. 1 7. 1 4. 37 181 427 0. 87 74. 6 84. 8 11 62. 3 7. 8 5. 1 4. 41 186 515 0. 91 65. 6 85. 4 12 59. 8 7. 8 2. 8 4. 22 182 510 0. 87 47. 3 89. 0 13 59. 1 9. 0 8. 0 4. 03 146 462 0. 93 63. 7 77. 9 14 59. 3 8. 0 10. 4 4. 13 158 624 0. 91 75. 1 84. 6 15 60. 9 8. 2 10. 2 4. 11 194 443 0. 89 79. 4 74. 3 16 60. 8 9. 8 10. 1 11. 94 175 314 0. 91 80. 8 93. 3 17 59. 0 12. 4 11. 4 13. 78 181 358 0. 9 74. 6 97. 4 18 60. 6 10. 7 12. 1 14. 36 168 377 0. 87 76. 7 99. 3 19 59. 8 7. 7 12. 1 4. 08 190 386 0. 9 86. 8 73. 6 20 61. 8 7. 7 10. 2 4. 13 185 398 0. 9 83. 2 78. 6 21

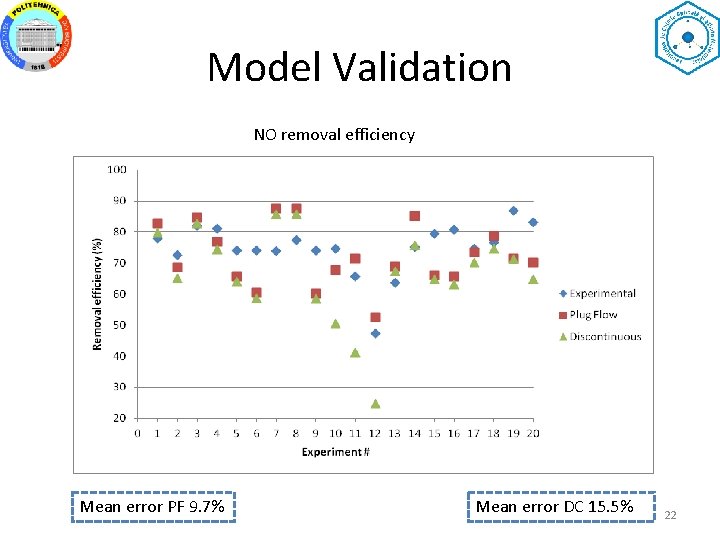
Model Validation NO removal efficiency Mean error PF 9. 7% Mean error DC 15. 5% 22

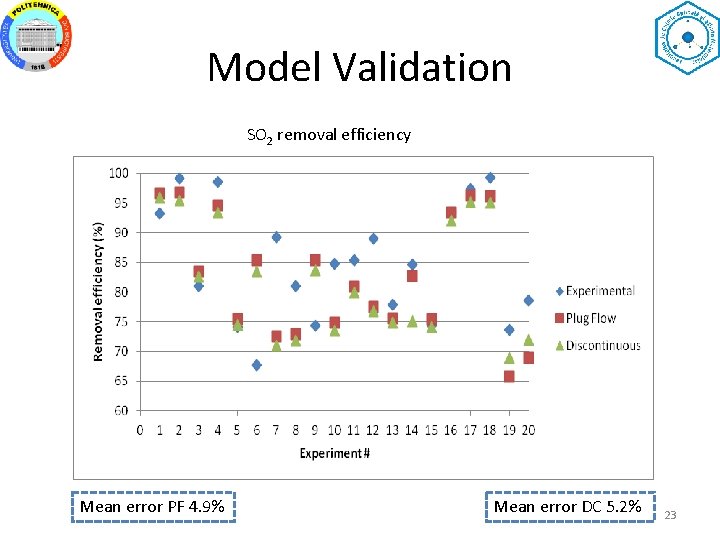
Model Validation SO 2 removal efficiency Mean error PF 4. 9% Mean error DC 5. 2% 23

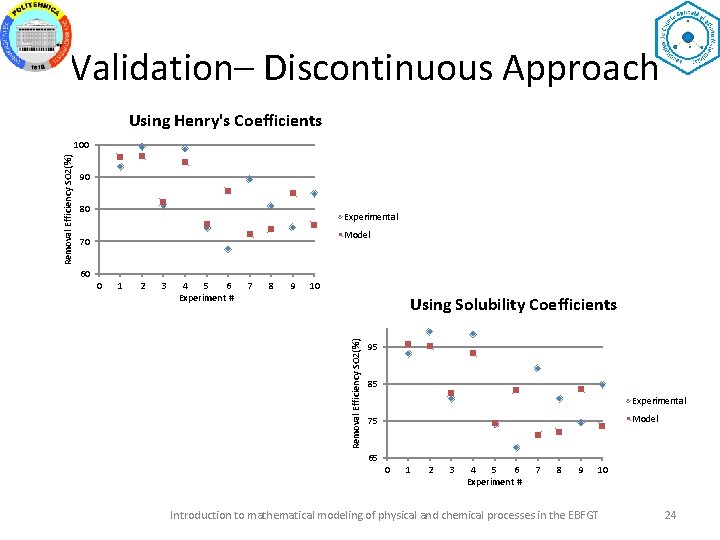
Validation– Discontinuous Approach Using Henry's Coefficients 90 80 Experimental Model 70 60 0 1 2 3 4 5 6 Experiment # 7 8 9 10 Using Solubility Coefficients Removal Efficiency SO 2(%) 100 95 85 Experimental Model 75 65 0 1 2 3 4 5 6 Experiment # 7 8 9 10 Introduction to mathematical modeling of physical and chemical processes in the EBFGT 24

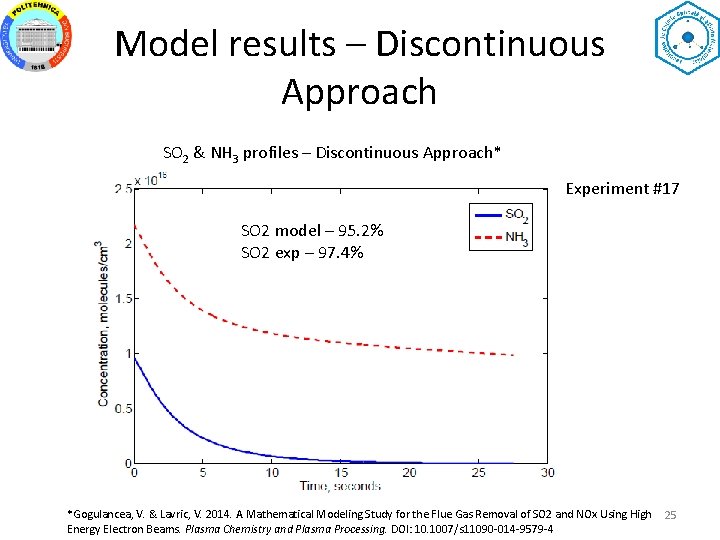
Model results – Discontinuous Approach SO 2 & NH 3 profiles – Discontinuous Approach* Experiment #17 SO 2 model – 95. 2% SO 2 exp – 97. 4% *Gogulancea, V. & Lavric, V. 2014. A Mathematical Modeling Study for the Flue Gas Removal of SO 2 and NOx Using High Energy Electron Beams. Plasma Chemistry and Plasma Processing. DOI: 10. 1007/s 11090 -014 -9579 -4 25

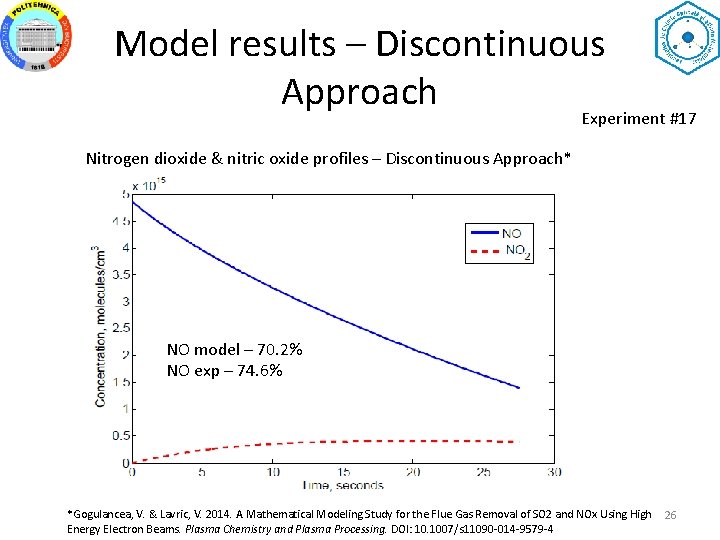
Model results – Discontinuous Approach Experiment #17 Nitrogen dioxide & nitric oxide profiles – Discontinuous Approach* NO model – 70. 2% NO exp – 74. 6% *Gogulancea, V. & Lavric, V. 2014. A Mathematical Modeling Study for the Flue Gas Removal of SO 2 and NOx Using High Energy Electron Beams. Plasma Chemistry and Plasma Processing. DOI: 10. 1007/s 11090 -014 -9579 -4 26

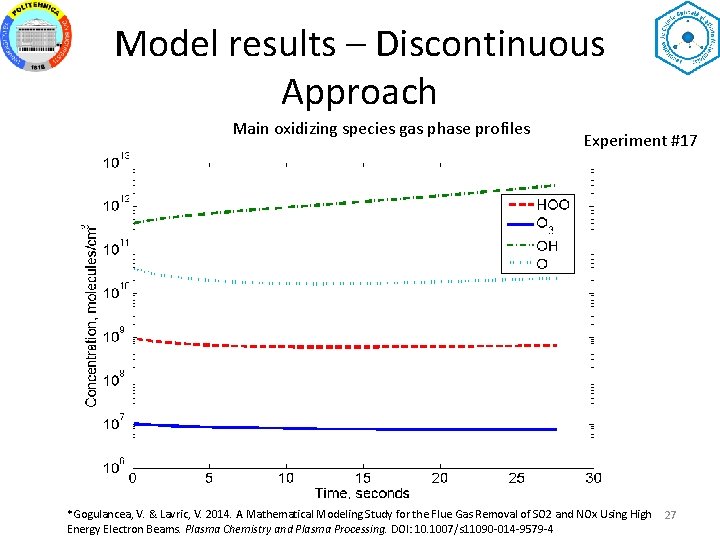
Model results – Discontinuous Approach Main oxidizing species gas phase profiles Experiment #17 *Gogulancea, V. & Lavric, V. 2014. A Mathematical Modeling Study for the Flue Gas Removal of SO 2 and NOx Using High Energy Electron Beams. Plasma Chemistry and Plasma Processing. DOI: 10. 1007/s 11090 -014 -9579 -4 27

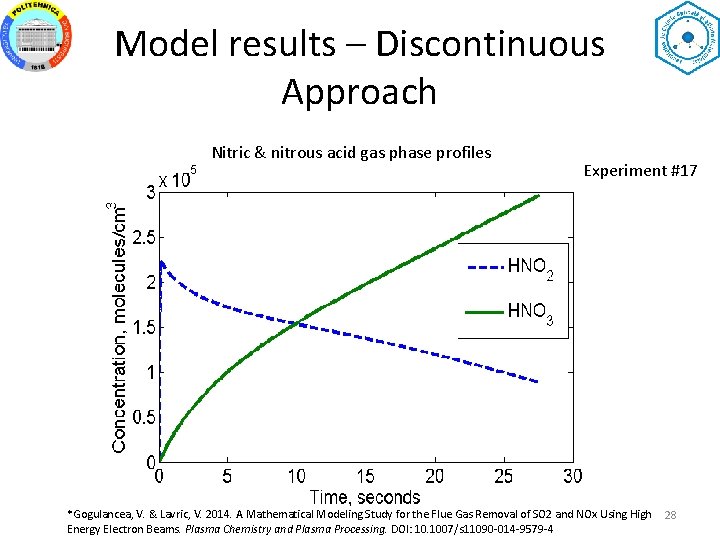
Model results – Discontinuous Approach Nitric & nitrous acid gas phase profiles Experiment #17 *Gogulancea, V. & Lavric, V. 2014. A Mathematical Modeling Study for the Flue Gas Removal of SO 2 and NOx Using High Energy Electron Beams. Plasma Chemistry and Plasma Processing. DOI: 10. 1007/s 11090 -014 -9579 -4 28

Model results – Discontinuous Approach Ammonia nitrate and sulfate profiles Experiment #17 *Gogulancea, V. & Lavric, V. 2014. A Mathematical Modeling Study for the Flue Gas Removal of SO 2 and NOx Using High Energy Electron Beams. Plasma Chemistry and Plasma Processing. DOI: 10. 1007/s 11090 -014 -9579 -4 29

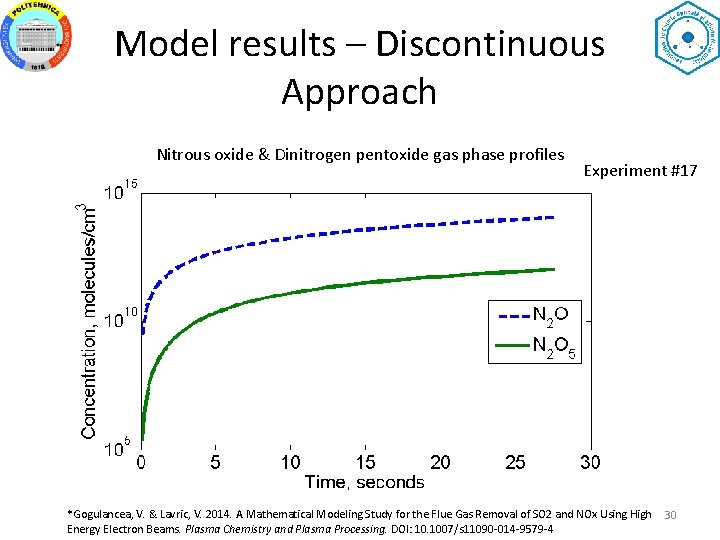
Model results – Discontinuous Approach Nitrous oxide & Dinitrogen pentoxide gas phase profiles Experiment #17 *Gogulancea, V. & Lavric, V. 2014. A Mathematical Modeling Study for the Flue Gas Removal of SO 2 and NOx Using High Energy Electron Beams. Plasma Chemistry and Plasma Processing. DOI: 10. 1007/s 11090 -014 -9579 -4 30

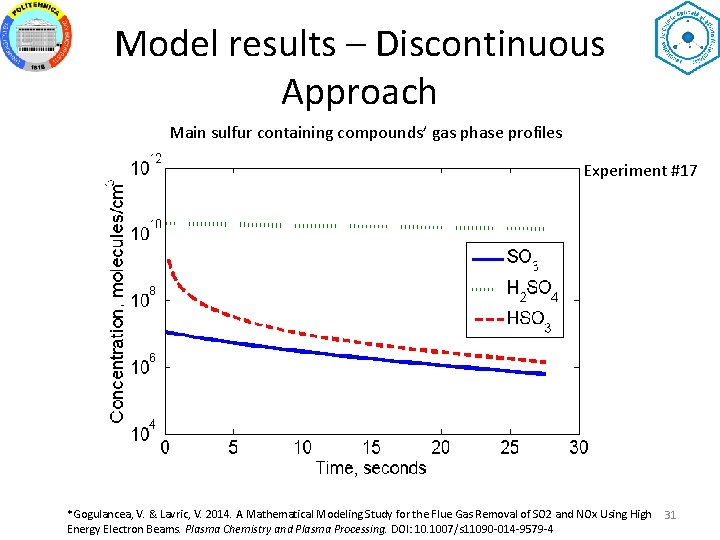
Model results – Discontinuous Approach Main sulfur containing compounds’ gas phase profiles Experiment #17 *Gogulancea, V. & Lavric, V. 2014. A Mathematical Modeling Study for the Flue Gas Removal of SO 2 and NOx Using High Energy Electron Beams. Plasma Chemistry and Plasma Processing. DOI: 10. 1007/s 11090 -014 -9579 -4 31

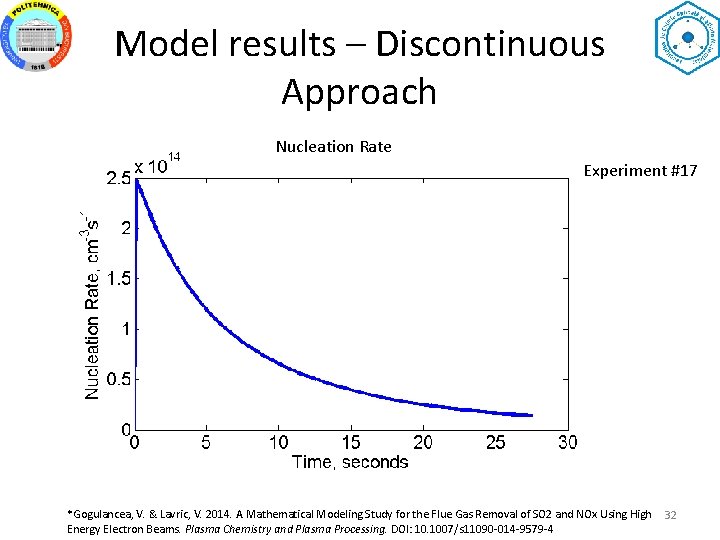
Model results – Discontinuous Approach Nucleation Rate Experiment #17 *Gogulancea, V. & Lavric, V. 2014. A Mathematical Modeling Study for the Flue Gas Removal of SO 2 and NOx Using High Energy Electron Beams. Plasma Chemistry and Plasma Processing. DOI: 10. 1007/s 11090 -014 -9579 -4 32

Model results – Discontinuous Approach Liquid phase profiles for the bisulfate & sulfate anions Experiment #17 *Gogulancea, V. & Lavric, V. 2014. A Mathematical Modeling Study for the Flue Gas Removal of SO 2 and NOx Using High Energy Electron Beams. Plasma Chemistry and Plasma Processing. DOI: 10. 1007/s 11090 -014 -9579 -4 33

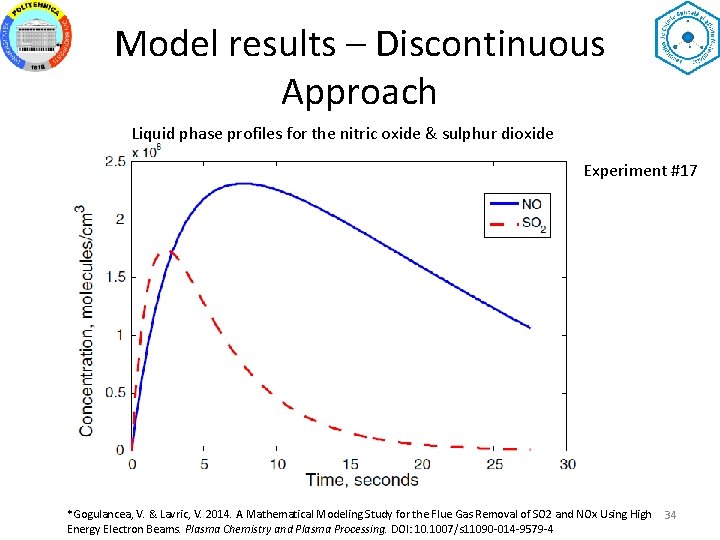
Model results – Discontinuous Approach Liquid phase profiles for the nitric oxide & sulphur dioxide Experiment #17 *Gogulancea, V. & Lavric, V. 2014. A Mathematical Modeling Study for the Flue Gas Removal of SO 2 and NOx Using High Energy Electron Beams. Plasma Chemistry and Plasma Processing. DOI: 10. 1007/s 11090 -014 -9579 -4 34

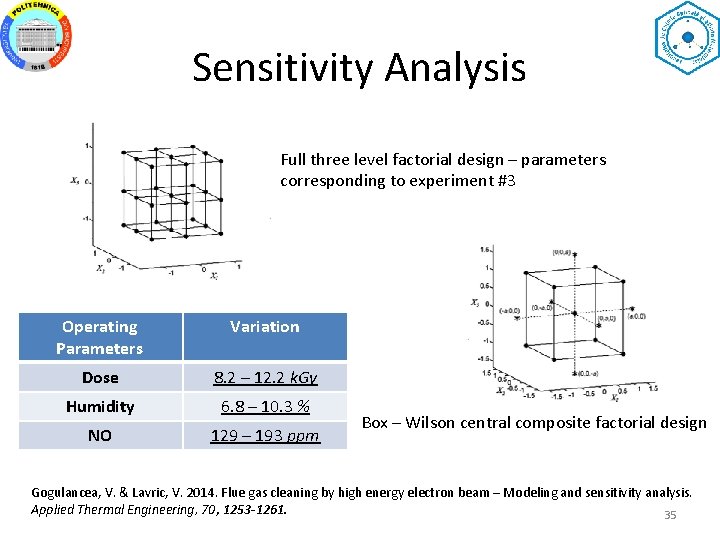
Sensitivity Analysis Full three level factorial design – parameters corresponding to experiment #3 Operating Parameters Variation Dose 8. 2 – 12. 2 k. Gy Humidity 6. 8 – 10. 3 % NO 129 – 193 ppm Box – Wilson central composite factorial design Gogulancea, V. & Lavric, V. 2014. Flue gas cleaning by high energy electron beam – Modeling and sensitivity analysis. Applied Thermal Engineering, 70, 1253 -1261. 35

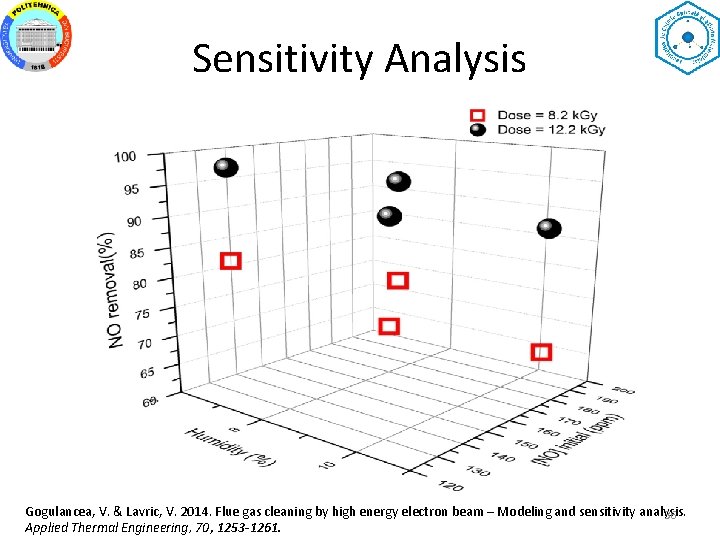
Sensitivity Analysis Gogulancea, V. & Lavric, V. 2014. Flue gas cleaning by high energy electron beam – Modeling and sensitivity analysis. 36 Applied Thermal Engineering, 70, 1253 -1261.

Sensitivity Analysis Gogulancea, V. & Lavric, V. 2014. Flue gas cleaning by high energy electron beam – Modeling and sensitivity analysis. 37 Applied Thermal Engineering, 70, 1253 -1261.

Perspectives • Fine water droplet addition • Optimization – response surface using ANN • Cost analysis • Relax the hypothesis of uniform dose distribution • Influence of axial dispersion – reactor configuration Introduction to mathematical modeling of physical and chemical processes in the EBFGT 38

Thank you for your attention! 39
 Helen erickson biography
Helen erickson biography Mathematical modeling and engineering problem solving
Mathematical modeling and engineering problem solving Mathematical modeling and engineering problem solving
Mathematical modeling and engineering problem solving Dimensional modeling vs relational modeling
Dimensional modeling vs relational modeling Mechanical system modeling examples
Mechanical system modeling examples Econ213
Econ213 Computational engineering and physical modeling
Computational engineering and physical modeling Conceptual physical and mathematical models are used to
Conceptual physical and mathematical models are used to Introduction to modeling and simulation
Introduction to modeling and simulation Pengertian pemodelan dan simulasi
Pengertian pemodelan dan simulasi Introduction and mathematical concepts
Introduction and mathematical concepts Introduction and mathematical concepts
Introduction and mathematical concepts Compare and contrast chemical and physical changes
Compare and contrast chemical and physical changes Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Modern chemistry chapter 7 review answers
Modern chemistry chapter 7 review answers Introduction to the unified modeling language
Introduction to the unified modeling language Introduction to unified modeling language
Introduction to unified modeling language Mercer oneview login
Mercer oneview login Properties of sulphuric acid
Properties of sulphuric acid Ethan is observing chemical and physical properties
Ethan is observing chemical and physical properties Whats the difference between physical and chemical change
Whats the difference between physical and chemical change Ideal properties of dental materials
Ideal properties of dental materials Physical change
Physical change Physical and chemical changes
Physical and chemical changes Physical property examples
Physical property examples Physical and chemical phenomena
Physical and chemical phenomena Is rocket fuel burning a physical change
Is rocket fuel burning a physical change Chemical changes
Chemical changes Physical and chemical changes generation genius
Physical and chemical changes generation genius Physical l change
Physical l change Physical change and chemical change
Physical change and chemical change What is weathering and its types
What is weathering and its types Differentiate between chemical and physical weathering.
Differentiate between chemical and physical weathering. Example of chemical weathering
Example of chemical weathering Chemical change grade 10
Chemical change grade 10 Difference between chemical and physical property
Difference between chemical and physical property Physical and chemical properties of helium
Physical and chemical properties of helium Whats the difference between chemical and physical change
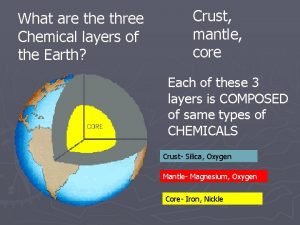
Whats the difference between chemical and physical change What are the three chemical layers of earth
What are the three chemical layers of earth Which is true about chemical change
Which is true about chemical change