Introduction to Materials Science and Engineering Course Overview



- Slides: 20

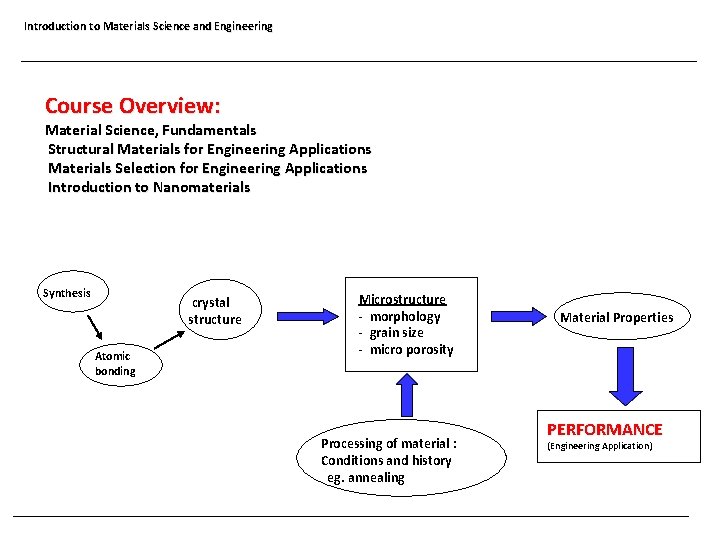
Introduction to Materials Science and Engineering Course Overview: Material Science, Fundamentals Structural Materials for Engineering Applications Materials Selection for Engineering Applications Introduction to Nanomaterials Synthesis crystal structure Atomic bonding Microstructure - morphology - grain size - micro porosity Processing of material : Conditions and history eg. annealing Material Properties PERFORMANCE (Engineering Application)

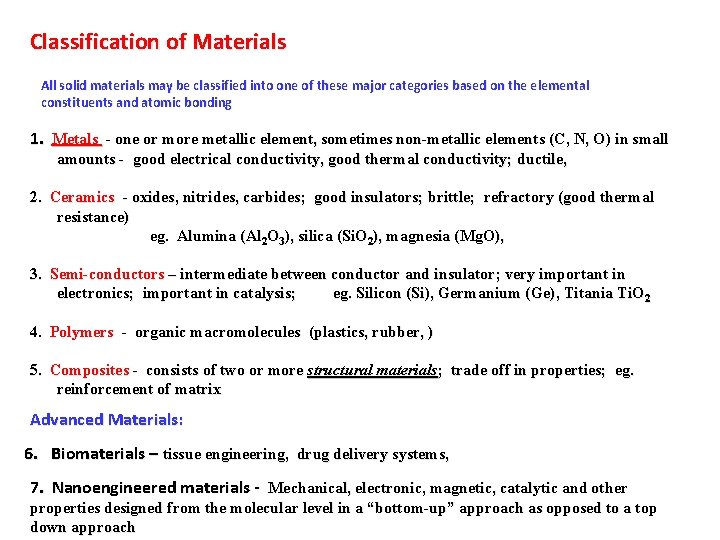
Classification of Materials All solid materials may be classified into one of these major categories based on the elemental constituents and atomic bonding 1. Metals - one or more metallic element, sometimes non-metallic elements (C, N, O) in small amounts - good electrical conductivity, good thermal conductivity; ductile, 2. Ceramics - oxides, nitrides, carbides; good insulators; brittle; refractory (good thermal resistance) eg. Alumina (Al 2 O 3), silica (Si. O 2), magnesia (Mg. O), 3. Semi-conductors – intermediate between conductor and insulator; very important in electronics; important in catalysis; eg. Silicon (Si), Germanium (Ge), Titania Ti. O 2 4. Polymers - organic macromolecules (plastics, rubber, ) 5. Composites - consists of two or more structural materials; trade off in properties; eg. reinforcement of matrix Advanced Materials: 6. Biomaterials – tissue engineering, drug delivery systems, 7. Nanoengineered materials - Mechanical, electronic, magnetic, catalytic and other properties designed from the molecular level in a “bottom-up” approach as opposed to a top down approach

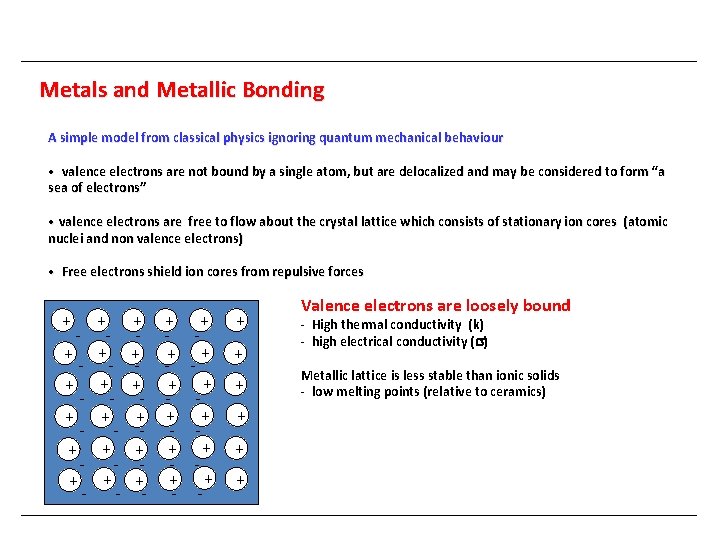
Metals and Metallic Bonding A simple model from classical physics ignoring quantum mechanical behaviour • valence electrons are not bound by a single atom, but are delocalized and may be considered to form “a sea of electrons” • valence electrons are free to flow about the crystal lattice which consists of stationary ion cores (atomic nuclei and non valence electrons) • Free electrons shield ion cores from repulsive forces + + - + + - + + - - - + + + - - Valence electrons are loosely bound - High thermal conductivity (k) - high electrical conductivity ( ) Metallic lattice is less stable than ionic solids - low melting points (relative to ceramics)

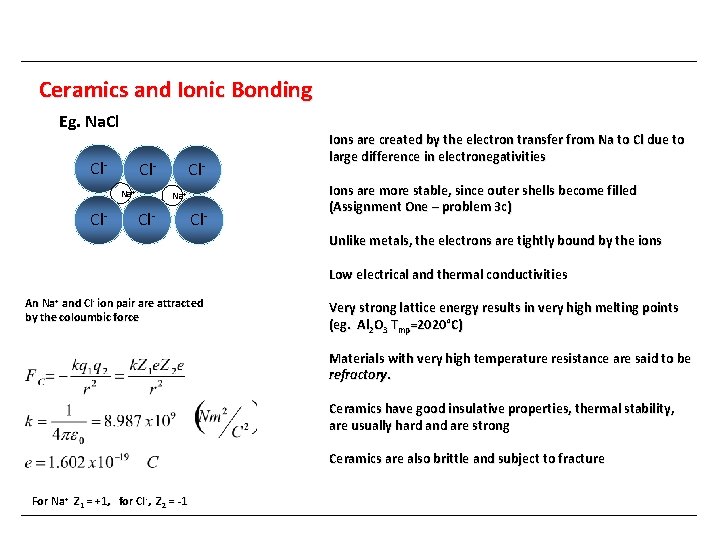
Ceramics and Ionic Bonding Eg. Na. Cl Cl- Cl. Na+ Cl- Ions are created by the electron transfer from Na to Cl due to large difference in electronegativities Ions are more stable, since outer shells become filled (Assignment One – problem 3 c) Unlike metals, the electrons are tightly bound by the ions Low electrical and thermal conductivities An Na+ and Cl- ion pair are attracted by the coloumbic force Very strong lattice energy results in very high melting points (eg. Al 2 O 3 Tmp=2020°C) Materials with very high temperature resistance are said to be refractory. Ceramics have good insulative properties, thermal stability, are usually hard and are strong Ceramics are also brittle and subject to fracture For Na+ Z 1 = +1, for Cl -, Z 2 = -1

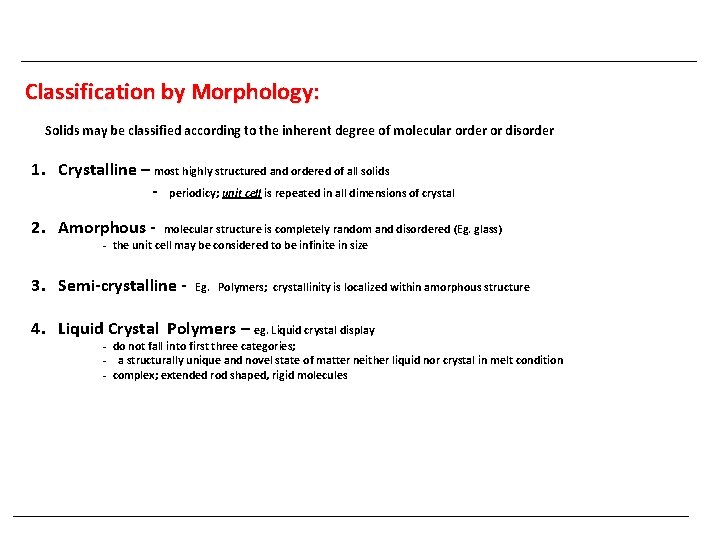
Classification by Morphology: Solids may be classified according to the inherent degree of molecular order or disorder 1. Crystalline – most highly structured and ordered of all solids - periodicy; unit cell is repeated in all dimensions of crystal 2. Amorphous - molecular structure is completely random and disordered (Eg. glass) - the unit cell may be considered to be infinite in size 3. Semi-crystalline - Eg. Polymers; crystallinity is localized within amorphous structure 4. Liquid Crystal Polymers – eg. Liquid crystal display - do not fall into first three categories; - a structurally unique and novel state of matter neither liquid nor crystal in melt condition - complex; extended rod shaped, rigid molecules

So, • Performance depends on material properties • Macroscopic material properties are governed by the microstructure • The microstructure is determined by crystal structure • Crystal structure is determined by atomic bonding • Classification of solids by atomic bonding and elemental constituents Metals, ceramics, semiconductors, polymers, composites

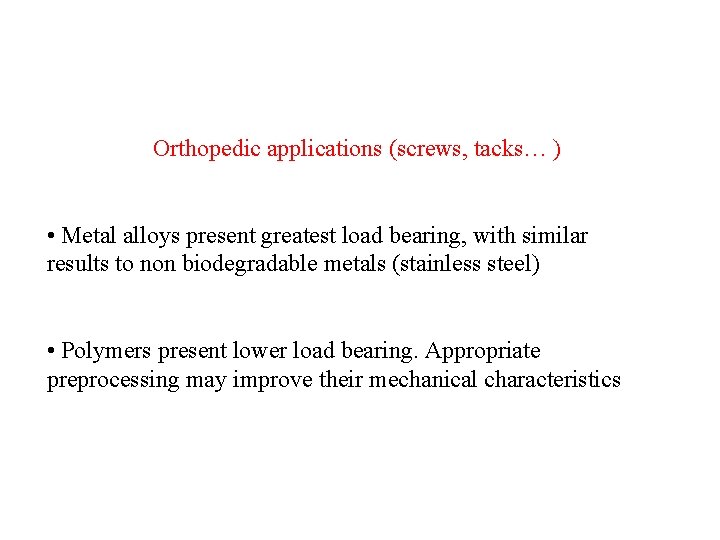
Orthopedic applications (screws, tacks… ) • Metal alloys present greatest load bearing, with similar results to non biodegradable metals (stainless steel) • Polymers present lower load bearing. Appropriate preprocessing may improve their mechanical characteristics

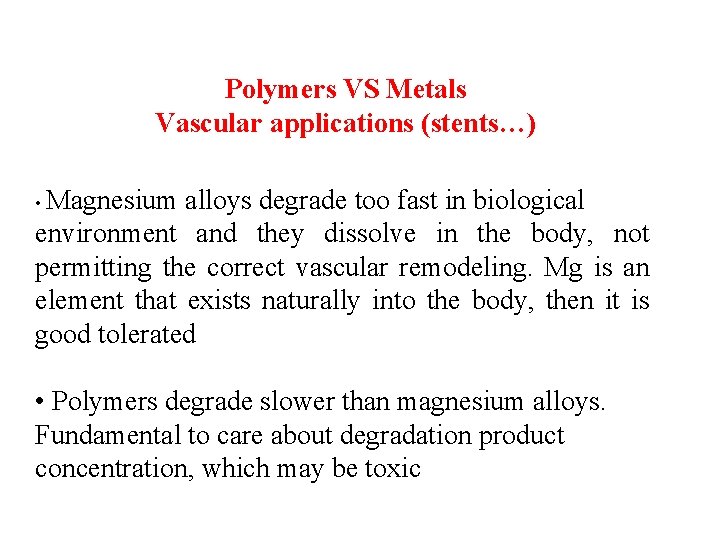
Polymers VS Metals Vascular applications (stents…) • Magnesium alloys degrade too fast in biological environment and they dissolve in the body, not permitting the correct vascular remodeling. Mg is an element that exists naturally into the body, then it is good tolerated • Polymers degrade slower than magnesium alloys. Fundamental to care about degradation product concentration, which may be toxic

Material Properties • OBJECTIVES • To introduce the fundamental mechanical and surface chemistry properties of biomaterials • OUTLINE – Mechanical Properties • elasticity, viscoelasticity, brittle fracture, fatigue – Surface chemistry

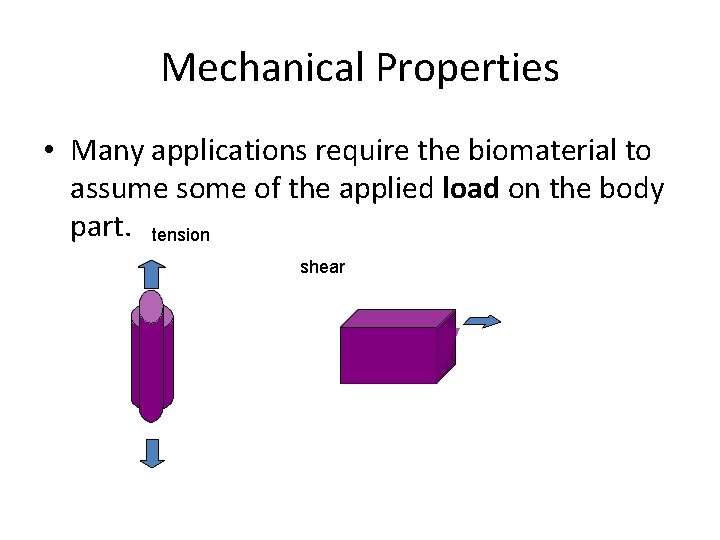
Mechanical Properties • Many applications require the biomaterial to assume some of the applied load on the body part. tension shear

Viscoelasticity • The response of materials to an imposed stress may under certain conditions resemble the behavior of a solid or a liquid. Stress Relaxation (application of a sudden strain to the sample and following the stress as a function of time as the strain is held constant). Creep (a constant stress is instantaneously applied to the material and the resulting strain is followed as a function of time)

Surface Energy • Interface – boundary between 2 layers • significance – protein adsorption to materials – blood coagulation/thrombosis due to material contact – cellular response to materials

Surface Chemistry • At the surface (interface) there are intermolecular forces and intramolecular forces of attraction and repulsion. B. Amsden CHEE 340

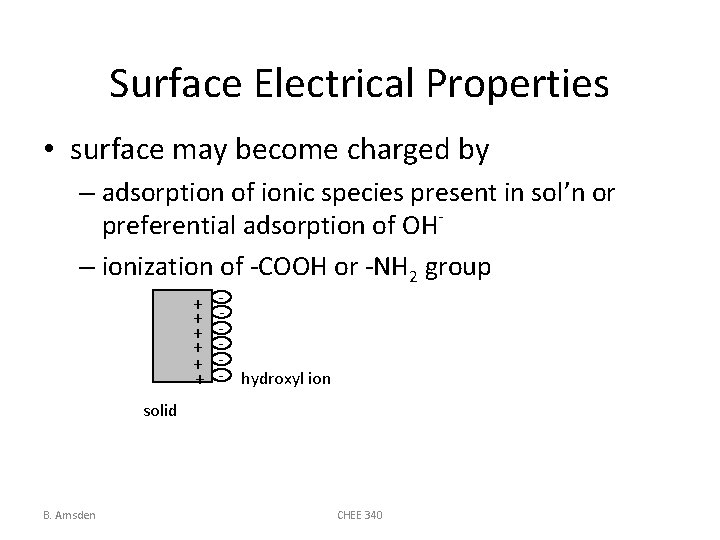
Surface Electrical Properties • surface may become charged by – adsorption of ionic species present in sol’n or preferential adsorption of OH– ionization of -COOH or -NH 2 group + + + - hydroxyl ion solid B. Amsden CHEE 340

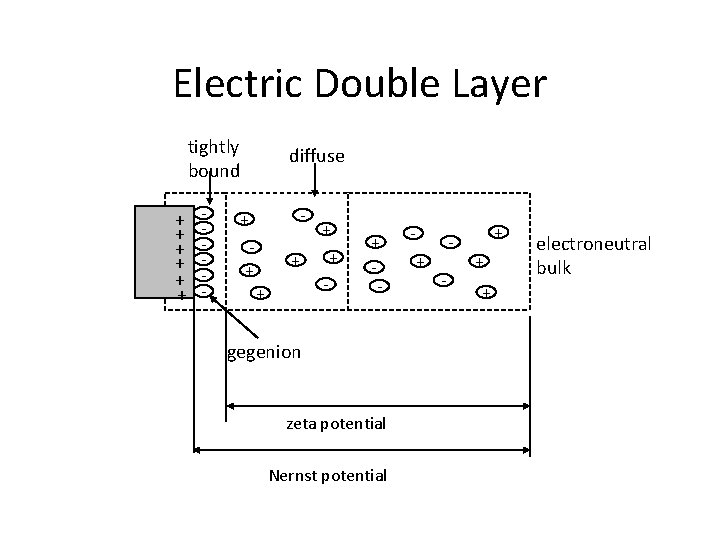
Electric Double Layer tightly bound + + + - diffuse - + + + - gegenion zeta potential Nernst potential + - + + + electroneutral bulk

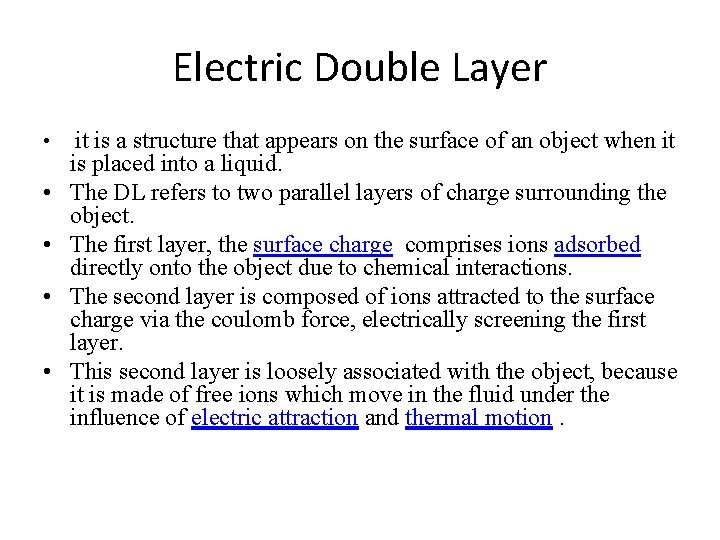
Electric Double Layer • • • it is a structure that appears on the surface of an object when it is placed into a liquid. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge comprises ions adsorbed directly onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the coulomb force, electrically screening the first layer. This second layer is loosely associated with the object, because it is made of free ions which move in the fluid under the influence of electric attraction and thermal motion.

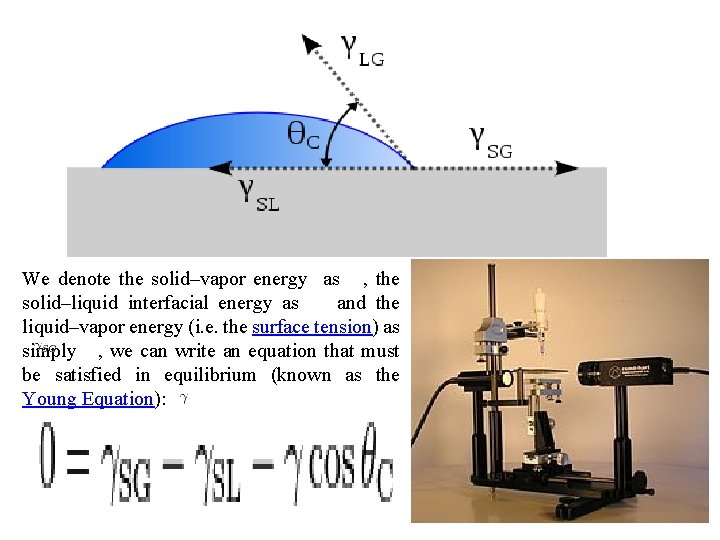
Surface Energy and the Contact Angle g. LV q g. SV g. SL

We denote the solid–vapor energy as , the solid–liquid interfacial energy as and the liquid–vapor energy (i. e. the surface tension) as simply , we can write an equation that must be satisfied in equilibrium (known as the Young Equation):

The contact angle can also be used to determine an interfacial energy (if other interfacial energies are known). This equation can be rewritten as the Young-Dupre equation: where W is the adhesion energy per unit area of the solid

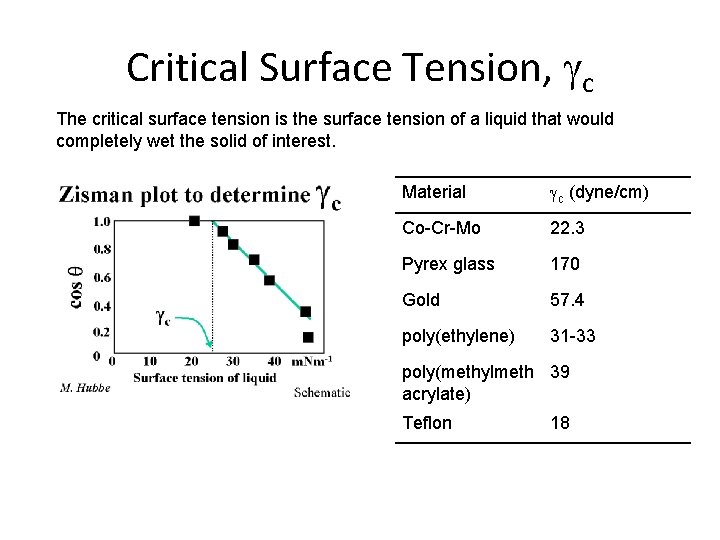
Critical Surface Tension, gc The critical surface tension is the surface tension of a liquid that would completely wet the solid of interest. Material gc (dyne/cm) Co-Cr-Mo 22. 3 Pyrex glass 170 Gold 57. 4 poly(ethylene) 31 -33 poly(methylmeth 39 acrylate) Teflon 18