Introduction to Limiting Reagents Learning Goals I will













- Slides: 20

Introduction to Limiting Reagents Learning Goals: I will be able to define what limiting and excess reagents are, and use them in a chemical equation to determine theoretical mass/moles produced, as well as be able to calculate the percent yield

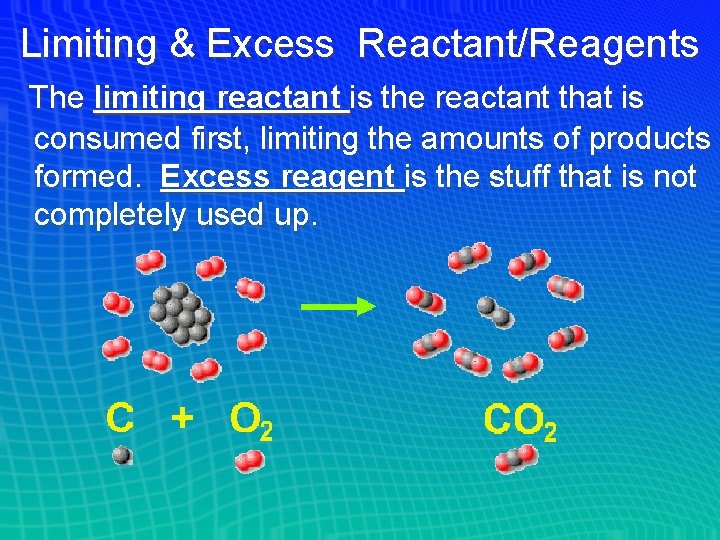
Limiting & Excess Reactant/Reagents The limiting reactant is the reactant that is consumed first, limiting the amounts of products formed. Excess reagent is the stuff that is not completely used up.

Limiting Reactant? I want to make chocolate chip cookies. I find in my kitchen: • 40 lbs. of butter • 80 lbs. of chocolate chips • 200 lbs. of flour • 150 lbs. of sugar • TWO eggs What is the limiting reagent? ? ?

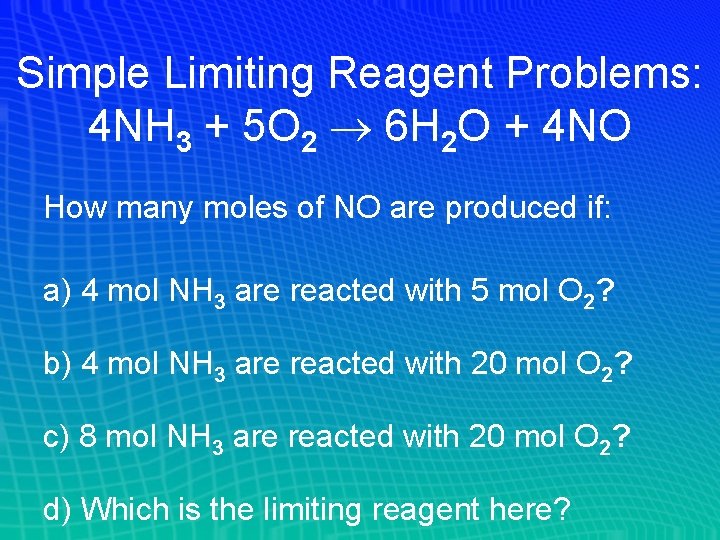
Simple Limiting Reagent Problems: 4 NH 3 + 5 O 2 6 H 2 O + 4 NO How many moles of NO are produced if: a) 4 mol NH 3 are reacted with 5 mol O 2? b) 4 mol NH 3 are reacted with 20 mol O 2? c) 8 mol NH 3 are reacted with 20 mol O 2? d) Which is the limiting reagent here?

Sample Questions: 2 A + 3 C D + 4 E a) If 3 moles of C is reacted with an excess of A, how many moles of D and E are produced? b) Example: How many moles of A are required to react completely with 4. 5 moles of C? c) You Try: How many moles of A are required to produce o. 55 moles of E?

Sample Questions: 2 A + 3 C D + 4 E a) If 3 moles of C is reacted with an excess of A, how many moles of D and E are produced? b) Example: How many moles of A are required to react completely with 4. 5 moles of C? c) You Try: How many moles of A are required to produce o. 55 moles of E?

5 H 2 SO 4 + 4 Zn 4 Zn. SO 4 + H 2 S + 4 H 2 O a) Example: If 3 moles of sulfuric acid is reacted with an excess of zinc, how much zinc sulfate would be produced? b) You Try: How many moles of water would be produced is 3. 8 moles of zinc was reacted with an excess of sulfuric acid? Complete: “The Mole and Chemical Calculations” worksheet

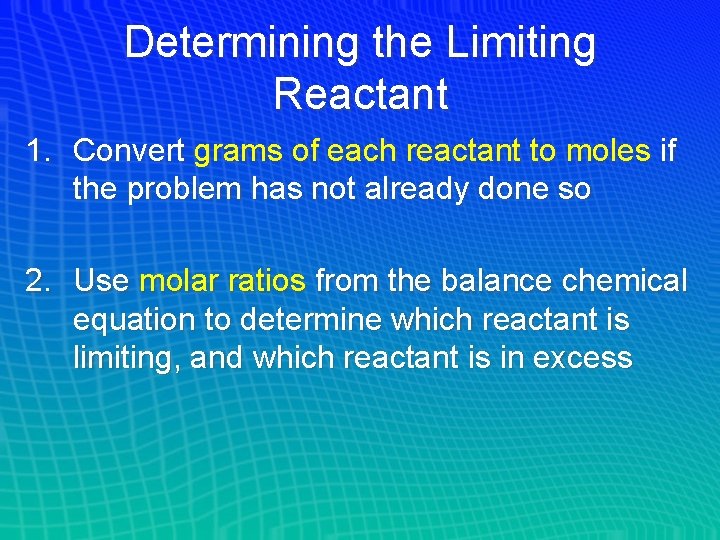
Determining the Limiting Reactant 1. Convert grams of each reactant to moles if the problem has not already done so 2. Use molar ratios from the balance chemical equation to determine which reactant is limiting, and which reactant is in excess

Example: 4 NH 3 + 5 O 2 6 H 2 O + 4 NO If you have 60 g of NH 3, and 75 g of O 2, which is the limiting reactant?

You Try 4 NH 3 + 5 O 2 6 H 2 O + 4 NO If you have 140 g of NH 3, and 180 g of O 2, which is the limiting reactant?

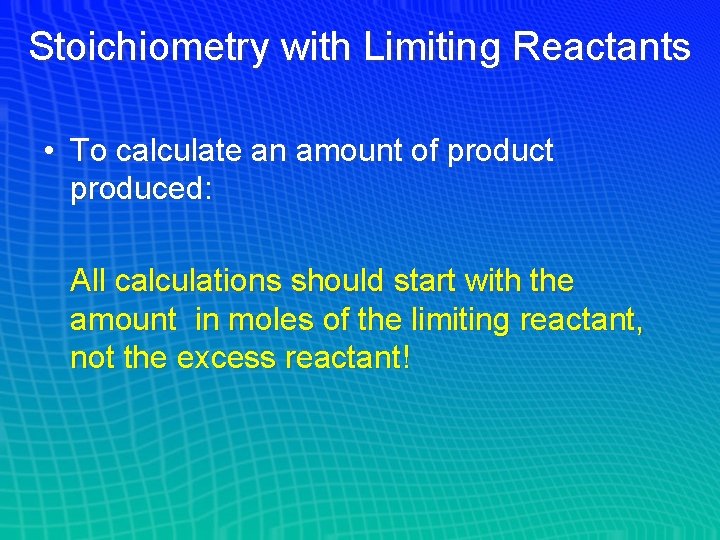
Stoichiometry with Limiting Reactants • To calculate an amount of product produced: All calculations should start with the amount in moles of the limiting reactant, not the excess reactant!

Example (moles to grams) 4 NH 3 + 5 O 2 6 H 2 O + 4 NO How many grams of NO are produced if 5 moles NH 3 are burned in 6 mol O 2?

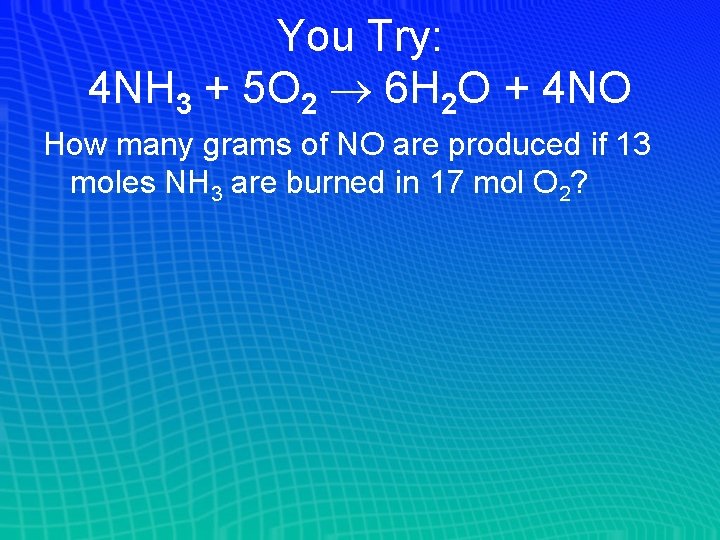
You Try: 4 NH 3 + 5 O 2 6 H 2 O + 4 NO How many grams of NO are produced if 13 moles NH 3 are burned in 17 mol O 2?

Example (grams to grams): 4 NH 3 + 5 O 2 6 H 2 O + 4 NO How many grams of NO are produced if 51 g of NH 3 are burned in 52 mol O 2?

You Try: 4 NH 3 + 5 O 2 6 H 2 O + 4 NO How many grams of NO are produced if 74 g of NH 3 are burned in 72 mol O 2?

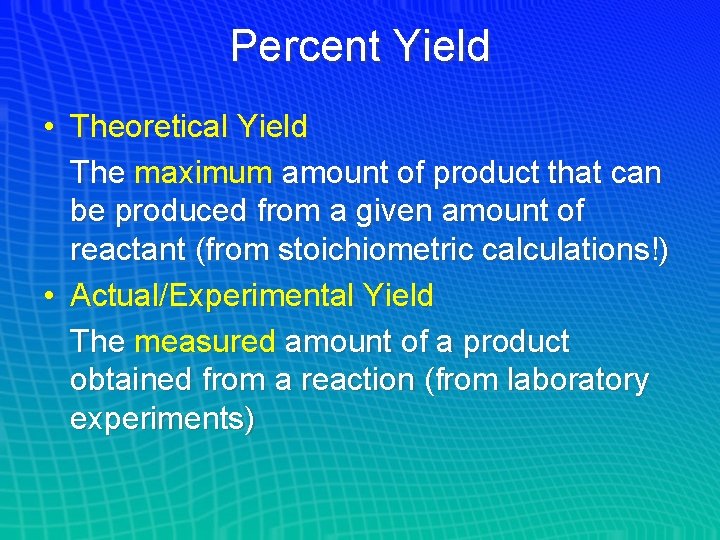
Percent Yield • Theoretical Yield The maximum amount of product that can be produced from a given amount of reactant (from stoichiometric calculations!) • Actual/Experimental Yield The measured amount of a product obtained from a reaction (from laboratory experiments)

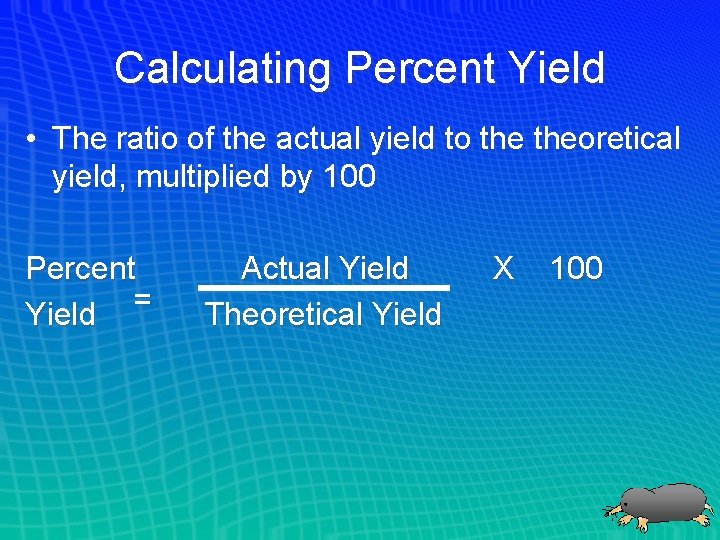
Calculating Percent Yield • The ratio of the actual yield to theoretical yield, multiplied by 100 Percent = Yield Actual Yield Theoretical Yield X 100

Example: 2 Mg + O 2 2 Mg. O 1. 65 g of Magnesium ribbon was burned in excess oxygen? Determine the percent of this reaction.

You Try: 2 Mg + O 2 2 Mg. O Magnesium ribbon was burned with excess oxygen in the rxn equation above. Using the data in the table below, what was the percent yield? Watch glass + tongs 273. 527 g Watch glass, tongs, Mg riibbon 273. 567 g Watch glass, tongs, product after burning 273. 564 g

How did we do? Learning Goals: I will be able to define what limiting and excess reagents are, and use them in a chemical equation to determine theoretical mass/moles produced, as well as be able to calculate the percent yield