INTRODUCTION TO LFD TESTING IN WORKPLACES Overview of


- Slides: 7

INTRODUCTION TO LFD TESTING IN WORKPLACES Overview of approach 8 February 2021

O F F I CI AL – S E N S I TI V E – C O M ME RCI AL Founding Principles 1 Testing is adaptable to the setting, as long as Social Distancing and safe practice is applied. The Guidebook covers settings of ALL sizes, but settings are all different (e. g. Testing a setting of 5 people is not the same as for one of 250+) 2 All Testing MUST be observed / supervised 3 All results MUST be recorded and registered via the Digital Solution as per the Guidebook 4 Employers are to maintain the twice weekly person regime wherever possible / practical to give the greatest degree of efficiency. 5 We are developing and learning together, in line with DHSC direction and guidance. It is collaborative and we will improve. 6 Further DHSC initiatives and schemes are being developed – however we are trying to enable you to Test, ensure the safety of your employees, and break chains of infection in this way NOW. 8

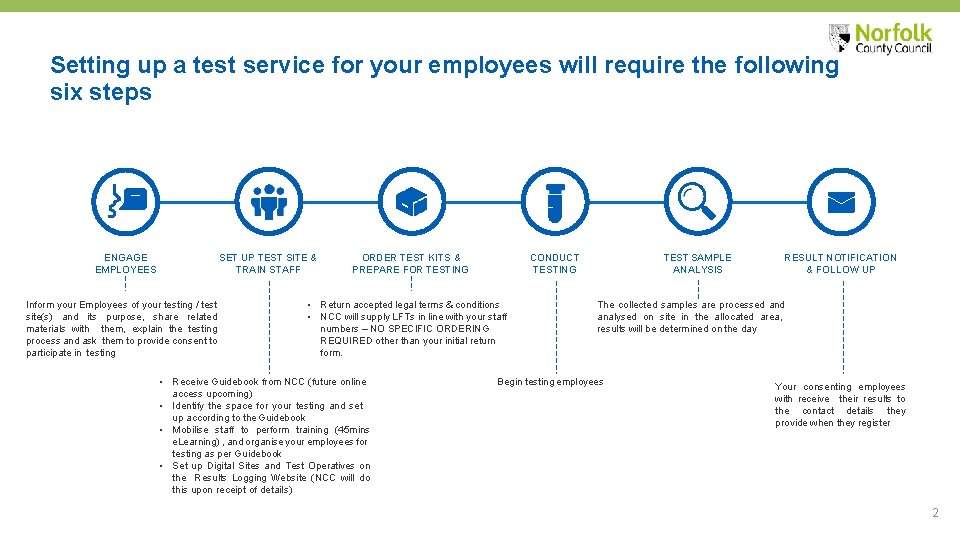
Setting up a test service for your employees will require the following six steps ENGAGE EMPLOYEES SET UP TEST SITE & TRAIN STAFF Inform your Employees of your testing / test site(s) and its purpose, share related materials with them, explain the testing process and ask them to provide consent to participate in testing ORDER TEST KITS & PREPARE FOR TESTING CONDUCT TESTING • Return accepted legal terms & conditions • NCC will supply LFTs in line with your staff numbers – NO SPECIFIC ORDERING REQUIRED other than your initial return form. • Receive Guidebook from NCC (future online access upcoming) • Identify the space for your testing and set up according to the Guidebook • Mobilise staff to perform training (45 mins e. Learning) , and organise your employees for testing as per Guidebook • Set up Digital Sites and Test Operatives on the Results Logging Website (NCC will do this upon receipt of details) TEST SAMPLE ANALYSIS RESULT NOTIFICATION & FOLLOW UP The collected samples are processed analysed on site in the allocated area, results will be determined on the day Begin testing employees Your consenting employees with receive their results to the contact details they provide when they register 2

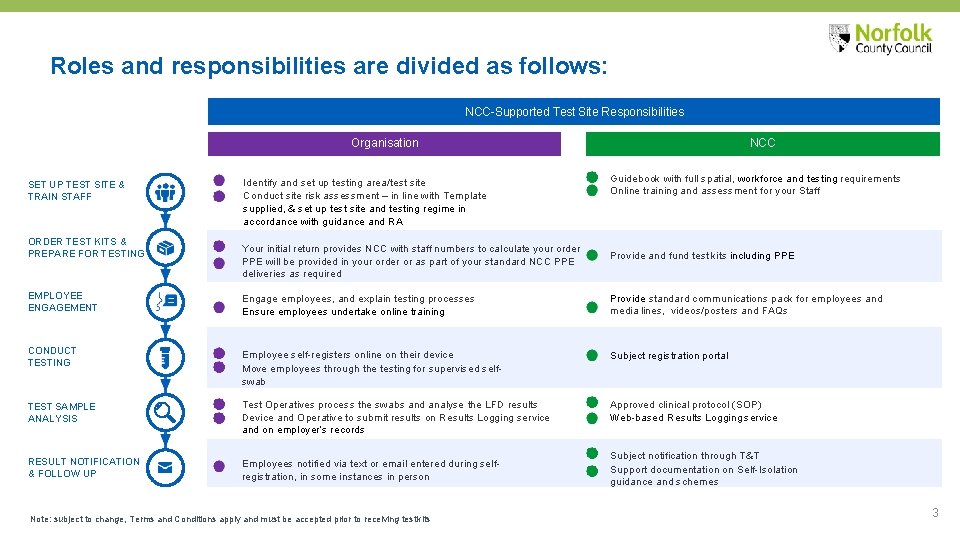
Roles and responsibilities are divided as follows: NCC-Supported Test Site Responsibilities Organisation SET UP TEST SITE & TRAIN STAFF ORDER TEST KITS & PREPARE FOR TESTING Identify and set up testing area/test site Conduct site risk assessment – in line with Template supplied, & set up test site and testing regime in accordance with guidance and RA Your initial return provides NCC with staff numbers to calculate your order PPE will be provided in your order or as part of your standard NCC PPE deliveries as required NCC Guidebook with full spatial, workforce and testing requirements Online training and assessment for your Staff Provide and fund test kits including PPE EMPLOYEE ENGAGEMENT Engage employees, and explain testing processes Ensure employees undertake online training Provide standard communications pack for employees and media lines, videos/posters and FAQs CONDUCT TESTING Employee self-registers online on their device Move employees through the testing for supervised selfswab Subject registration portal TEST SAMPLE ANALYSIS Test Operatives process the swabs and analyse the LFD results Device and Operative to submit results on Results Logging service and on employer’s records Approved clinical protocol (SOP) Web-based Results Logging service RESULT NOTIFICATION & FOLLOW UP Employees notified via text or email entered during selfregistration, in some instances in person Note: subject to change, Terms and Conditions apply and must be accepted prior to receiving test kits Subject notification through T&T Support documentation on Self-Isolation guidance and schemes 3

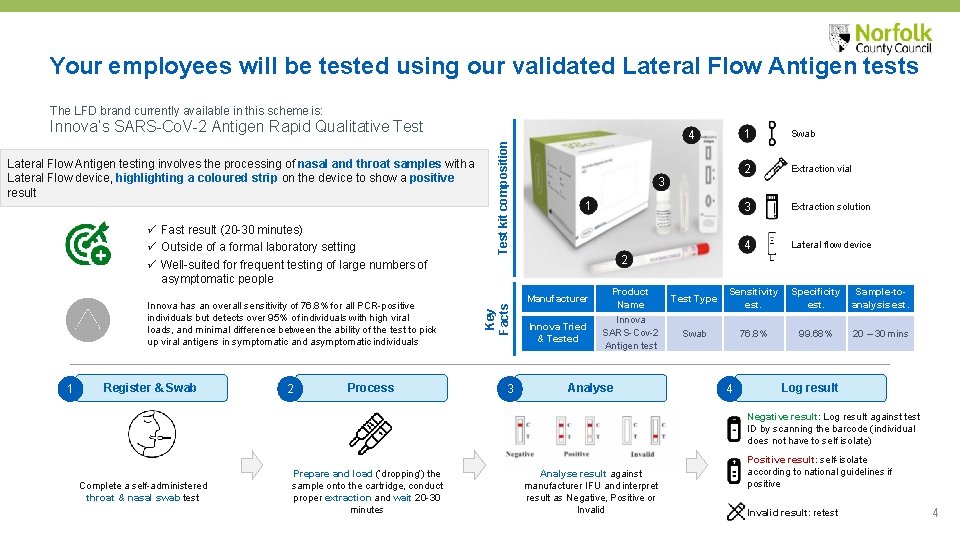
Your employees will be tested using our validated Lateral Flow Antigen tests The LFD brand currently available in this scheme is: Fast result (20 -30 minutes) Outside of a formal laboratory setting Well-suited for frequent testing of large numbers of asymptomatic people Innova has an overall sensitivity of 76. 8% for all PCR-positive individuals but detects over 95% of individuals with high viral loads, and minimal difference between the ability of the test to pick up viral antigens in symptomatic and asymptomatic individuals 1 Register & Swab 2 Process Key Facts Lateral Flow Antigen testing involves the processing of nasal and throat samples with a Lateral Flow device, highlighting a coloured strip on the device to show a positive result Test kit composition Innova’s SARS-Co. V-2 Antigen Rapid Qualitative Test 3 4 3 1 1 Swab 2 Extraction vial 3 Extraction solution 4 Lateral flow device 2 Manufacturer Product Name Test Type Sensitivity est. Specificity est. Sample-toanalysis est. Innova Tried & Tested Innova SARS-Cov-2 Antigen test Swab 76. 8% 99. 68% 20 – 30 mins Analyse 4 Log result Negative result: Log result against test ID by scanning the barcode (individual does not have to self isolate) Complete a self-administered throat & nasal swab test Prepare and load (‘dropping’) the sample onto the cartridge, conduct proper extraction and wait 20 -30 minutes Analyse result against manufacturer IFU and interpret result as Negative, Positive or Invalid Positive result: self-isolate according to national guidelines if positive Invalid result: retest 4

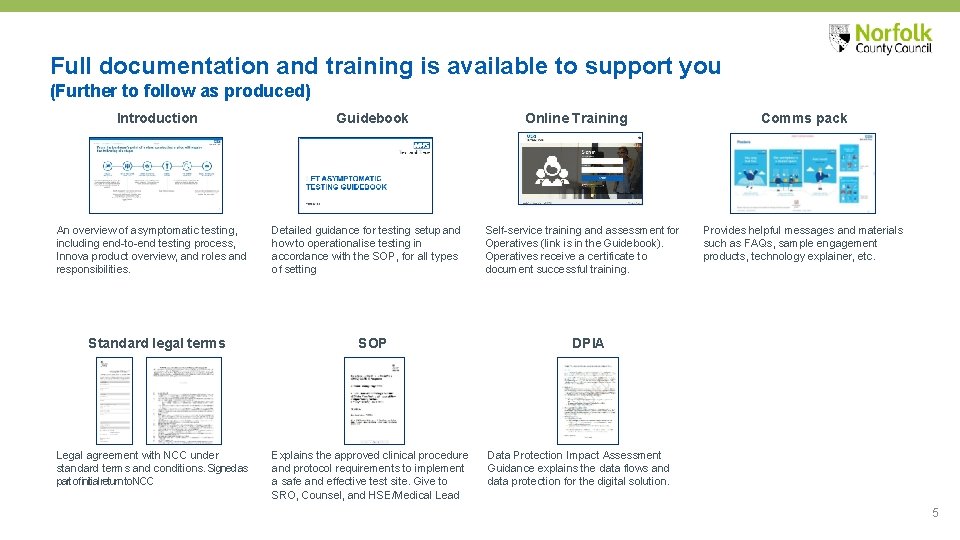
Full documentation and training is available to support you (Further to follow as produced) Introduction An overview of asymptomatic testing, including end-to-end testing process, Innova product overview, and roles and responsibilities. Standard legal terms Legal agreement with NCC under standard terms and conditions. Signed as part of initial return to NCC Guidebook Detailed guidance for testing setup and how to operationalise testing in accordance with the SOP, for all types of setting SOP Explains the approved clinical procedure and protocol requirements to implement a safe and effective test site. Give to SRO, Counsel, and HSE/Medical Lead Online Training Self-service training and assessment for Operatives (link is in the Guidebook). Operatives receive a certificate to document successful training. Comms pack Provides helpful messages and materials such as FAQs, sample engagement products, technology explainer, etc. DPIA Data Protection Impact Assessment Guidance explains the data flows and data protection for the digital solution. 5

Each organisation agrees to accept NCC’s Terms and Conditions which are underpinned by the following core assumptions Policy Commercial & Legal 1. Testing will not remove requirements to follow all national government guidance on COVID-safe workplaces, such as social distancing 6. 2. Those who test positive, and their close contacts, will need to self isolate as per government guidelines The organisation will construct and set up testing environments in accordance with SOP guidelines (including storage areas for tests) for sample collection, analysis, disposal and reporting 7. NCC will fund and supply the tests, subject to contractual agreement, for a limited period. All other associated costs will be funded by the employer. Operations Outcomes 3. Tests must be supervised by staff who will be given suitable training via an online platform 8. 4. Employees will be tested under a schedule of twice weekly. Testing will not be compulsory Test results will be shared with NPEx (National Pathology Exchange) prior to anonymised onward distribution to Public Health England (All Tests registered as per the Guidebook on the portal) 9. Under current legislation, employers do not receive results directly 5. Sample materials need to be treated as healthcare waste. Disposal will take place at the place of test, per SOP & Guidebook requirements Note: subject to change, Terms and Conditions apply and must be accepted prior to receiving test kits 6