Introduction to Geochronology Part 1 The basics Geochronology

Introduction to Geochronology Part 1: The basics Geochronology & Tracers Facility NERC Isotope Geosciences Laboratory British Geological Survey

Introduction to Geochronology • Pat 1: Geochronology – basic principles – P/D, decay constants, chronometers • Part 2: Framing the problem (1) – What and why? – sampling, targeting, minerals and meanings • Part 3: Framing the problem (2) - Petrochronology – imaging, trace elements, metamorphic examples • Part 4: Rocks into Ratios – mass spectrometry, traceability, dissolution chemistry • Part 5: Ratios into Dates – uncertainties, reporting, reading legacy data

Geochronology basics • Why geochronology? – calibration • Geological timescale • Events - mass extinctions Great Oxygenation Event Snowball Earth Age of the Earth and the Solar System • Astronomical clock, eustacy…
Geochronology basics • Why geochronology? – rates • Tectonic – plate to outcrop scales • Metamorphism and fluids • Evolution of life

Geochronology basics • What is geochronology? Wikipedia: Geochronology is the science of determining the age of rocks, fossils, and sediments using signatures inherent in the rocks themselves. Absolute geochronology can be accomplished through radioactive isotopes, whereas relative geochronology is provided by tools such as palaeomagnetism and stable isotope ratios.

Geochronology basics • What is geochronology? Determining the age at which a particular radioactive decay chain, or part of it, is set/reset/disturbed within a mineral. How?
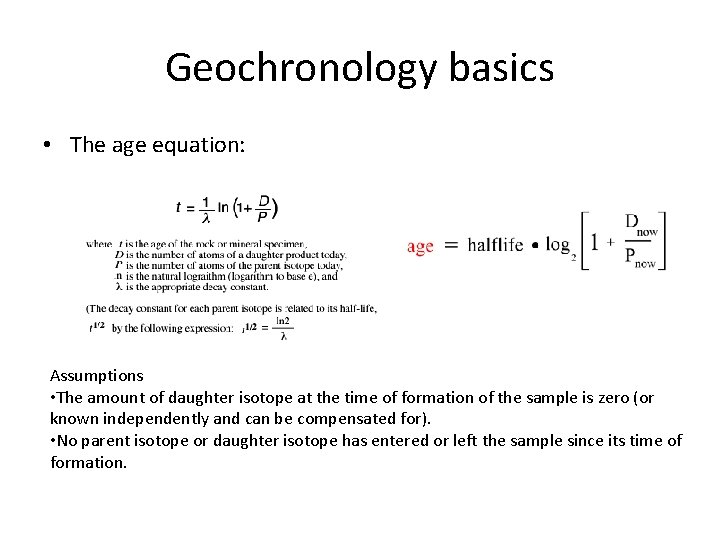
Geochronology basics • The age equation: Assumptions • The amount of daughter isotope at the time of formation of the sample is zero (or known independently and can be compensated for). • No parent isotope or daughter isotope has entered or left the sample since its time of formation.
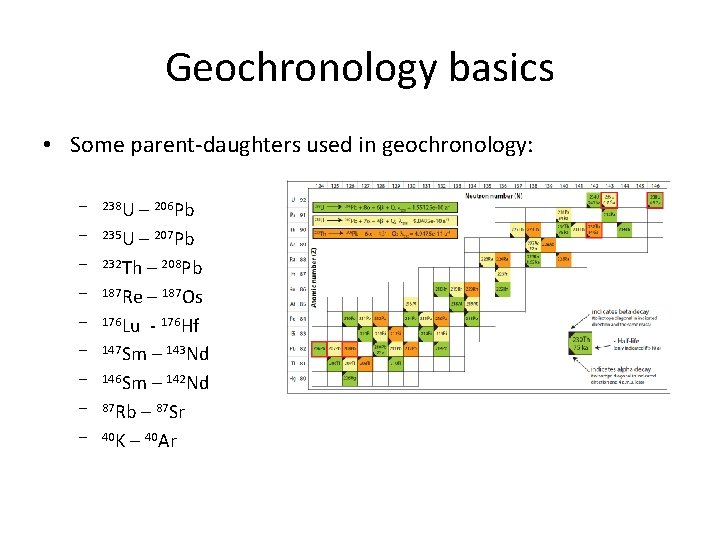
Geochronology basics • Some parent-daughters used in geochronology: – 238 U – 206 Pb – 235 U – 207 Pb – 232 Th – 208 Pb – 187 Re – 187 Os – 176 Lu - 176 Hf – 147 Sm – 143 Nd – 146 Sm – 142 Nd – 87 Rb – 87 Sr – 40 K – 40 Ar
Geochronology basics • What makes a good geochronometer? – Up-take of parent daughter during crystallisation – No up-take of daughter during crystallisation – Closed system to parent and daughter since crystallisation – i. e. high initial P/D ratio. Kd lattice-bound? diffusion
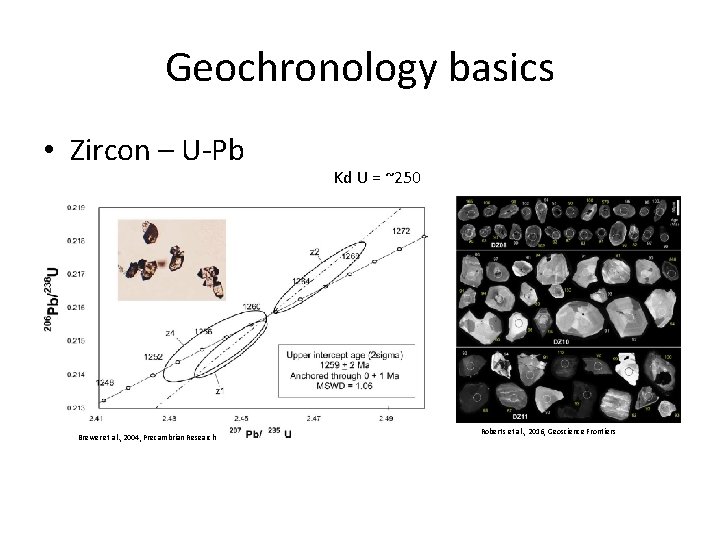
Geochronology basics • Zircon – U-Pb Brewer et al. , 2004, Precambrian Research Kd U = ~250 Roberts et al. , 2016, Geoscience Frontiers
Geochronology basics • Most chronometers are not perfect • Complications and limitations – – Incorporation of daughter (e. g. common lead) Inheritance of daughter (e. g. radiogenic precursor) Susceptibility to alteration Partial open-system behaviour
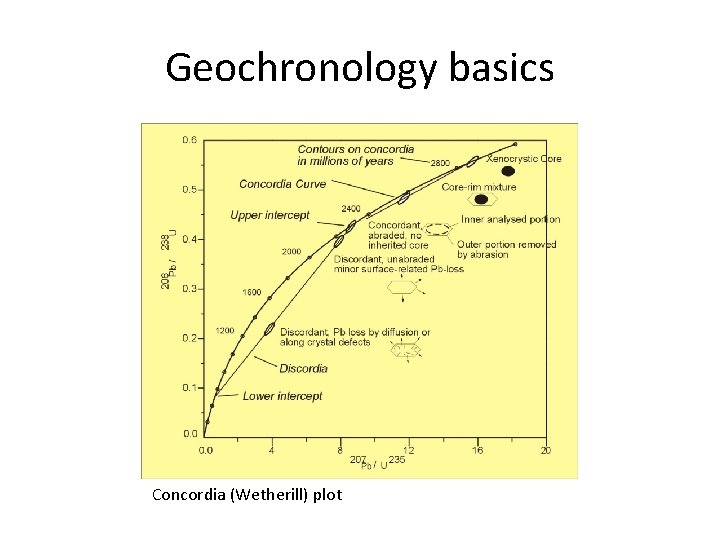
Geochronology basics Concordia (Wetherill) plot
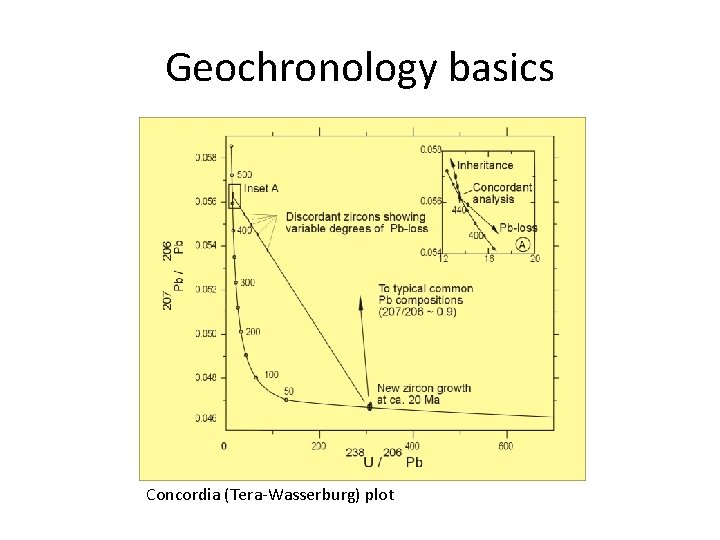
Geochronology basics Concordia (Tera-Wasserburg) plot
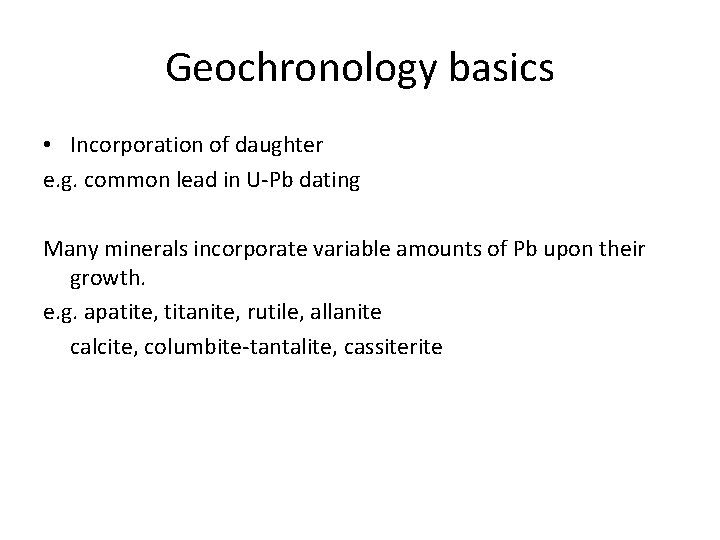
Geochronology basics • Incorporation of daughter e. g. common lead in U-Pb dating Many minerals incorporate variable amounts of Pb upon their growth. e. g. apatite, titanite, rutile, allanite calcite, columbite-tantalite, cassiterite

Geochronology basics • Incorporation of daughter e. g. common lead in U-Pb dating Rutile (Ti. O 2) Common-lead ad le n- o mm Co ID-TIMS U-Pb data for R 10 rutile Braccialli et al. , 2013, Chemical Geology LA U-Pb data for R 10
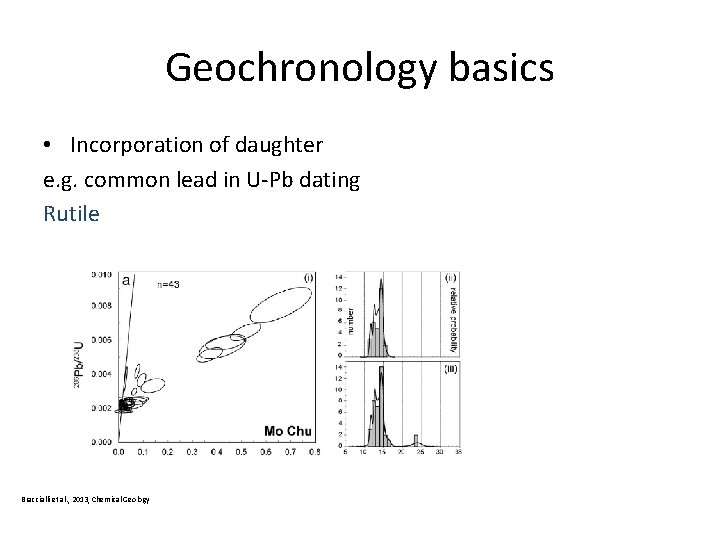
Geochronology basics • Incorporation of daughter e. g. common lead in U-Pb dating Rutile Braccialli et al. , 2013, Chemical Geology

Geochronology basics • Incorporation of daughter e. g. common lead in U-Pb dating Titanite (Ca. Ti. Si. O 5) common Pb radiogenic Pb Thomas et al. , 2013, Precambrian Research Data from Martin et al. , 2015, New Zealand Journal of Geology & Geophsyics
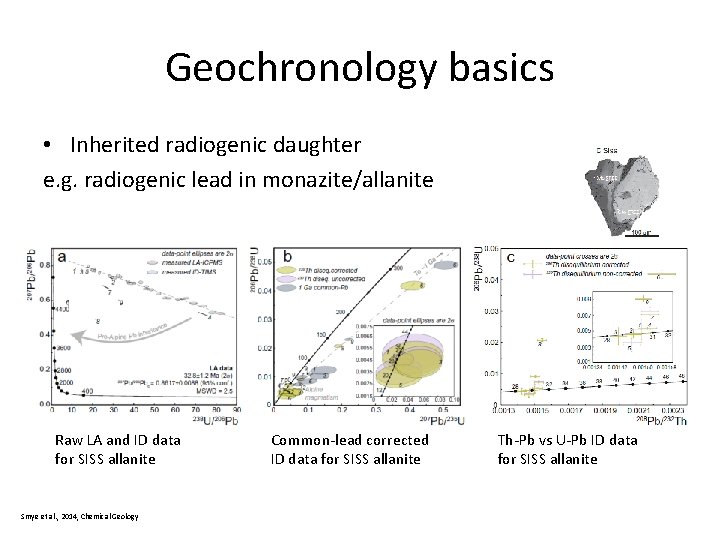
Geochronology basics • Inherited radiogenic daughter e. g. radiogenic lead in monazite/allanite Raw LA and ID data for SISS allanite Smye et al. , 2014, Chemical Geology Common-lead corrected ID data for SISS allanite Th-Pb vs U-Pb ID data for SISS allanite
Geochronology basics • Partially open-system – after metamictisation – above closure temperature – during fluid infiltration
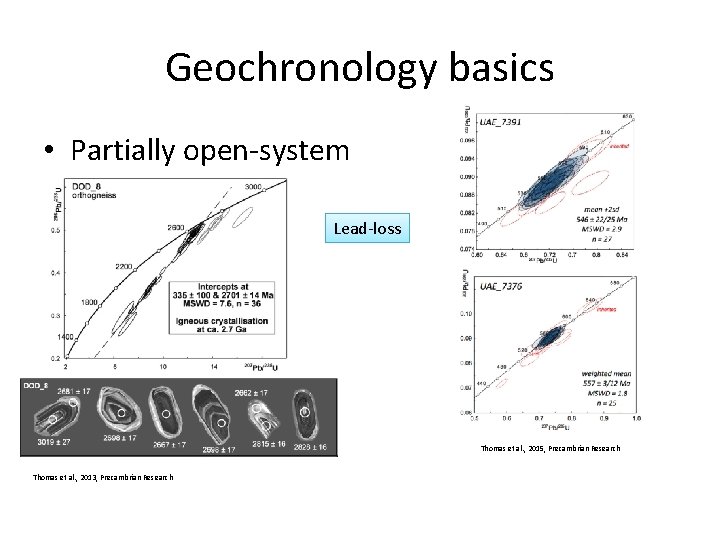
Geochronology basics • Partially open-system Lead-loss Thomas et al. , 2015, Precambrian Research Thomas et al. , 2013, Precambrian Research
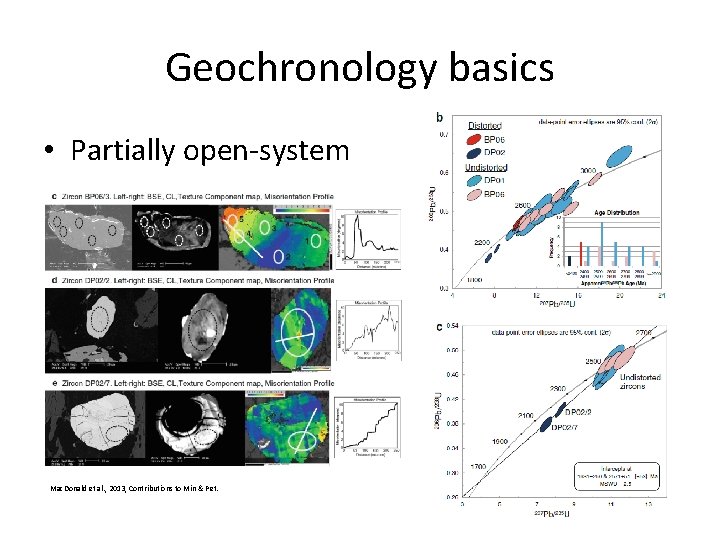
Geochronology basics • Partially open-system Mac. Donald et al. , 2013, Contributions to Min & Pet.
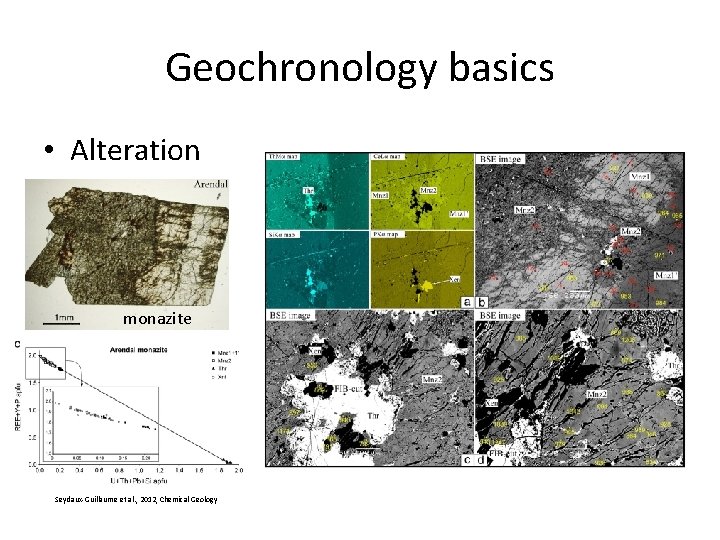
Geochronology basics • Alteration monazite Seydaux-Guillaume et al. , 2012, Chemical Geology
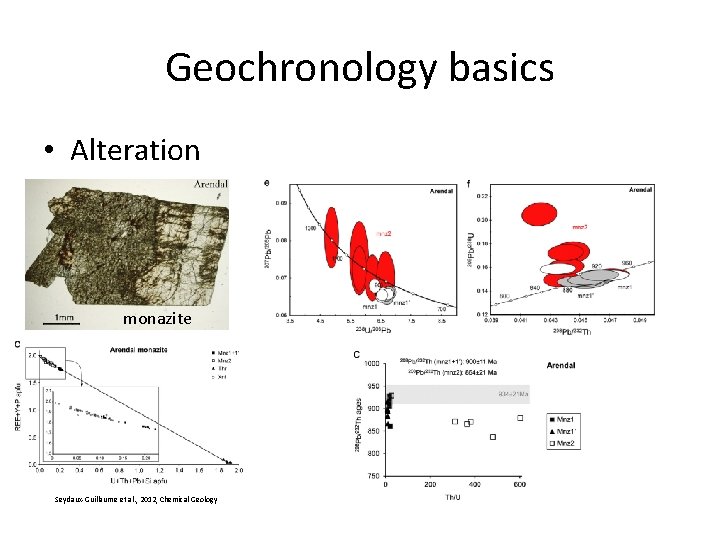
Geochronology basics • Alteration monazite Seydaux-Guillaume et al. , 2012, Chemical Geology
Geochronology basics • Most chronometers are not perfect • Complications and limitations – – Incorporation of daughter (e. g. common lead) Inheritance of daughter (e. g. radiogenic precursor) Susceptibility to alteration Partial open-system behaviour
Geochronology basics • Most chronometers are not perfect • Complications and limitations – – Incorporation of daughter (e. g. common lead) Inheritance of daughter (e. g. radiogenic precursor) Susceptibility to alteration Partial open-system behaviour Can we use any of this to our advantage?
Geochronology basics • Closure Temperature and Thermochronology – In ideal terms – the closure temperature is the temperature at the time given by the measured date of a mineral – The temperature at which a given parent-daughter decay chain is set, because no diffusion of the parent or daughter isotopes takes place at lower temperatures – The reality – closure temperature varies with grain size, and the rate of cooling.
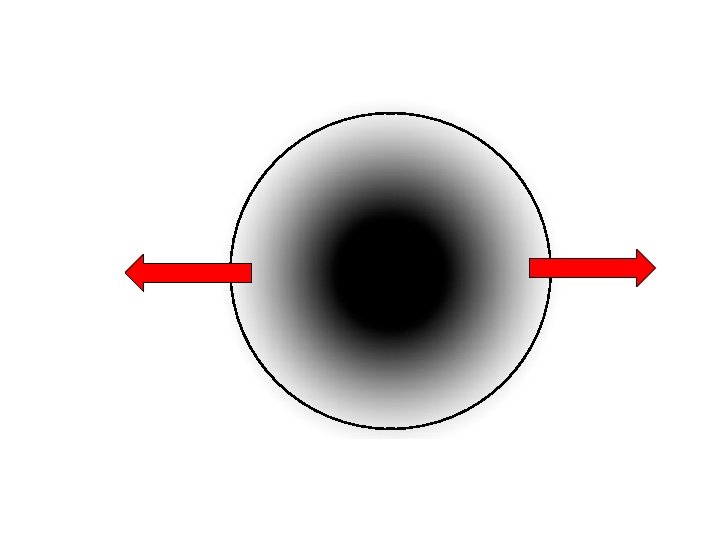
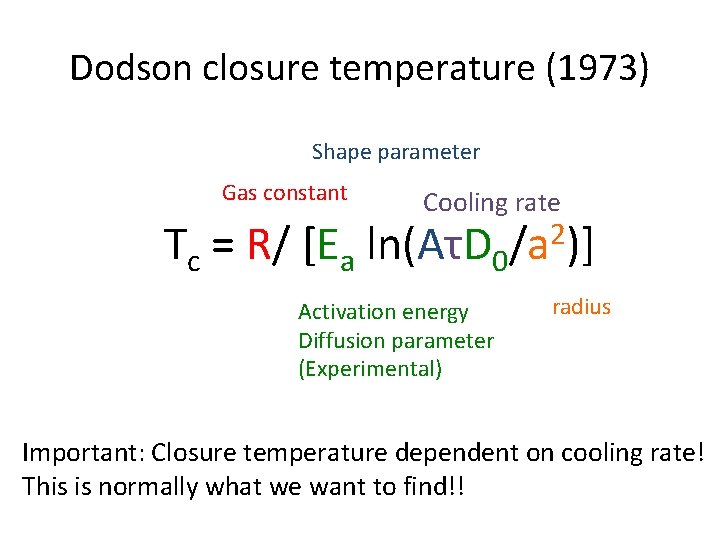
Dodson closure temperature (1973) Shape parameter Gas constant Cooling rate Tc = R/ [Ea ln(AτD 0/a 2)] Activation energy Diffusion parameter (Experimental) radius Important: Closure temperature dependent on cooling rate! This is normally what we want to find!!
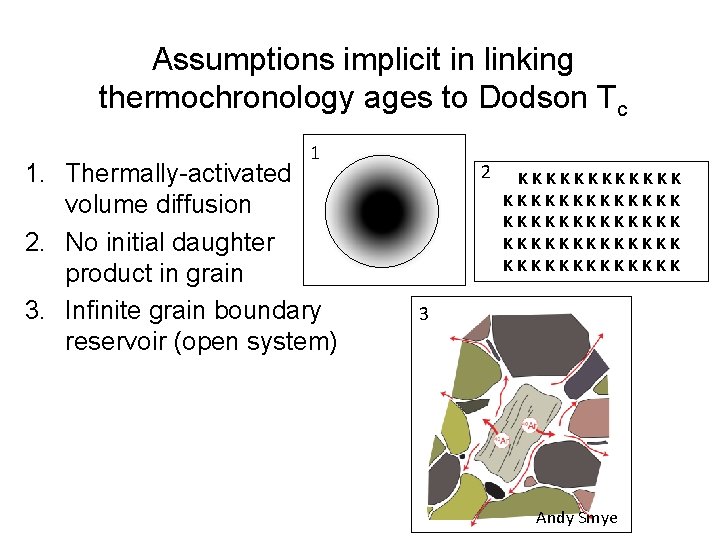
Assumptions implicit in linking thermochronology ages to Dodson Tc 1 1. Thermally-activated volume diffusion 2. No initial daughter product in grain 3. Infinite grain boundary reservoir (open system) 2 KKKKKKKKKKKKK KKKKKKK 3 Andy Smye
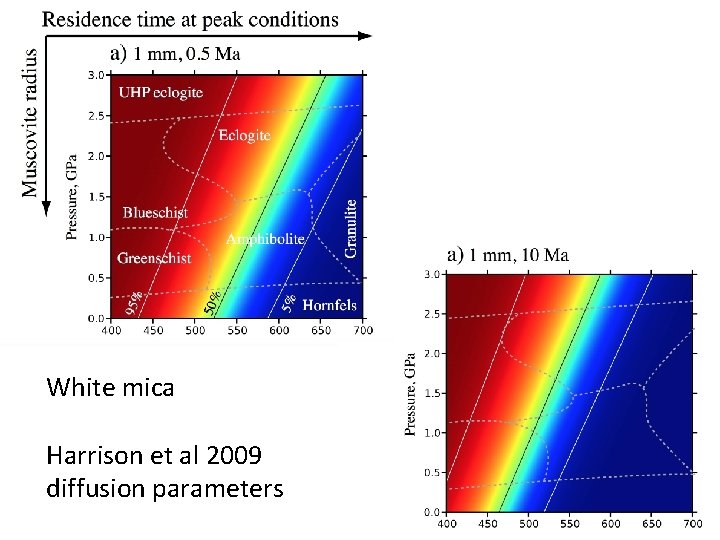
White mica Harrison et al 2009 diffusion parameters
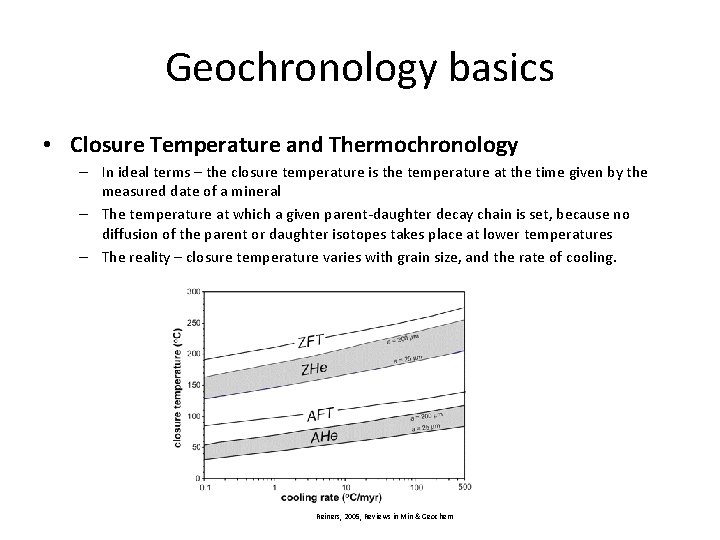
Geochronology basics • Closure Temperature and Thermochronology – In ideal terms – the closure temperature is the temperature at the time given by the measured date of a mineral – The temperature at which a given parent-daughter decay chain is set, because no diffusion of the parent or daughter isotopes takes place at lower temperatures – The reality – closure temperature varies with grain size, and the rate of cooling. Reiners, 2005, Reviews in Min & Geochem
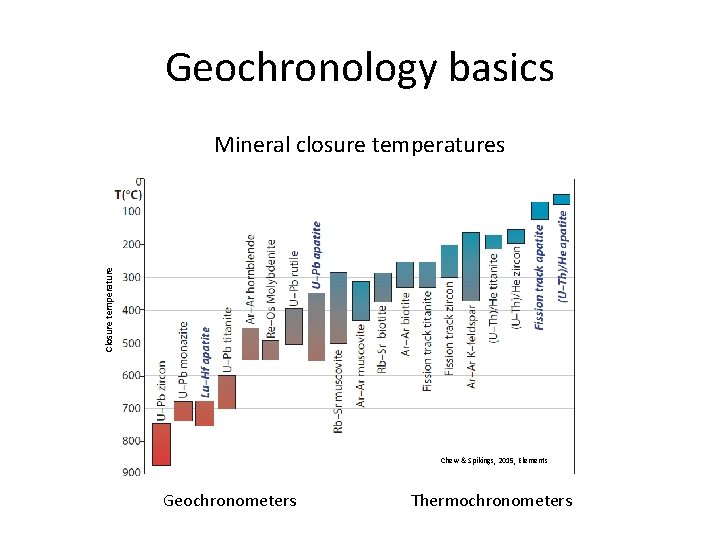
Geochronology basics Closure temperature Mineral closure temperatures Chew & Spikings, 2015, Elements Geochronometers Thermochronometers

Geochronology basics Pressure allanite garnet Crystallisation chronometers: Sm-Nd, Lu-Hf garnet U-Pb allanite, zircon, monazite zircon rutile titanite muscovite biotite Temperature Thermochronometers: Ar/Ar micas U-Th-He apatite, zircon Fission track apatite, zircon
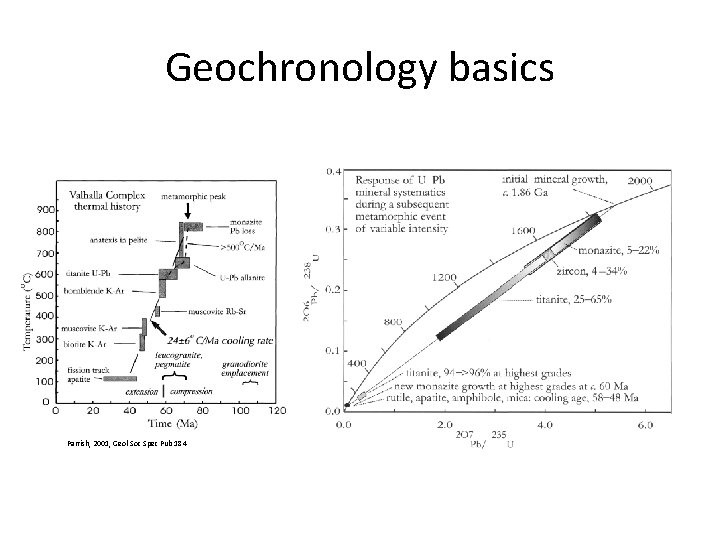
Geochronology basics Parrish, 2001, Geol Soc Spec Pub 184
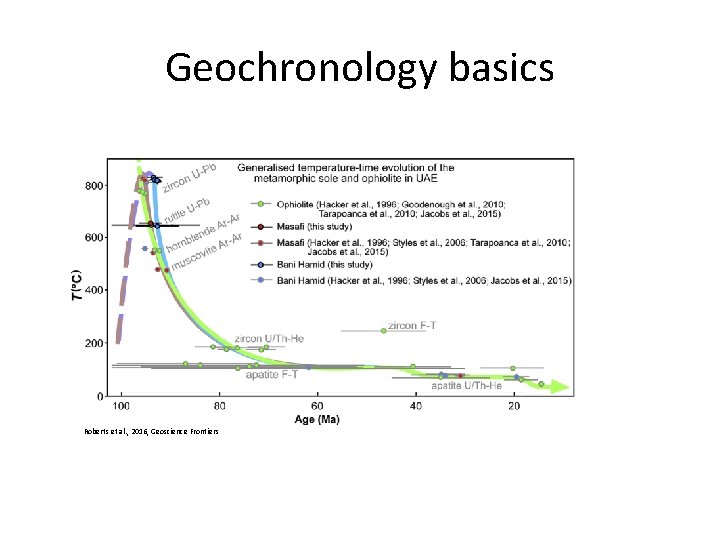
Geochronology basics Roberts et al. , 2016, Geoscience Frontiers
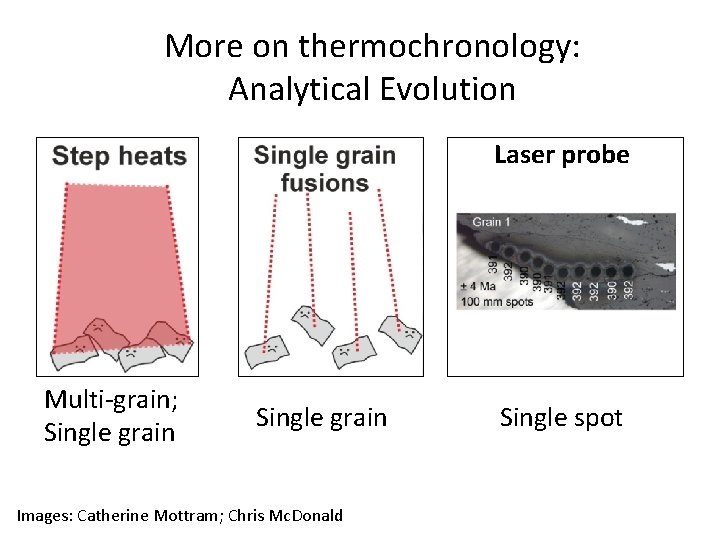
More on thermochronology: Analytical Evolution Laser probe Multi-grain; Single grain Images: Catherine Mottram; Chris Mc. Donald Single spot
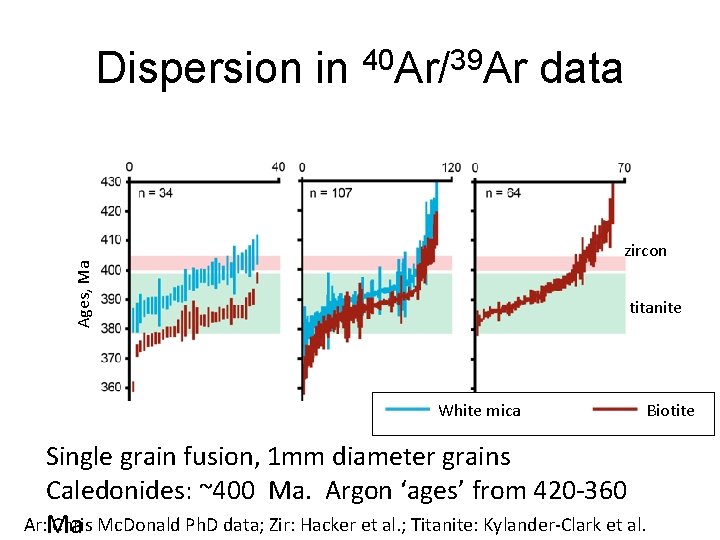
Dispersion in 40 Ar/39 Ar data Ages, Ma zircon titanite White mica Biotite Single grain fusion, 1 mm diameter grains Caledonides: ~400 Ma. Argon ‘ages’ from 420 -360 Ar: Chris Mc. Donald Ph. D data; Zir: Hacker et al. ; Titanite: Kylander-Clark et al. Ma
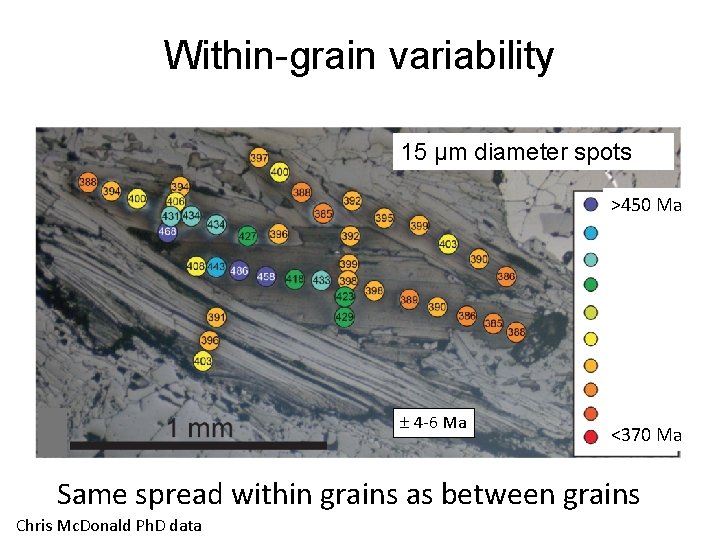
Within-grain variability 15 μm diameter spots >450 Ma 4 -6 Ma <370 Ma Same spread within grains as between grains Chris Mc. Donald Ph. D data
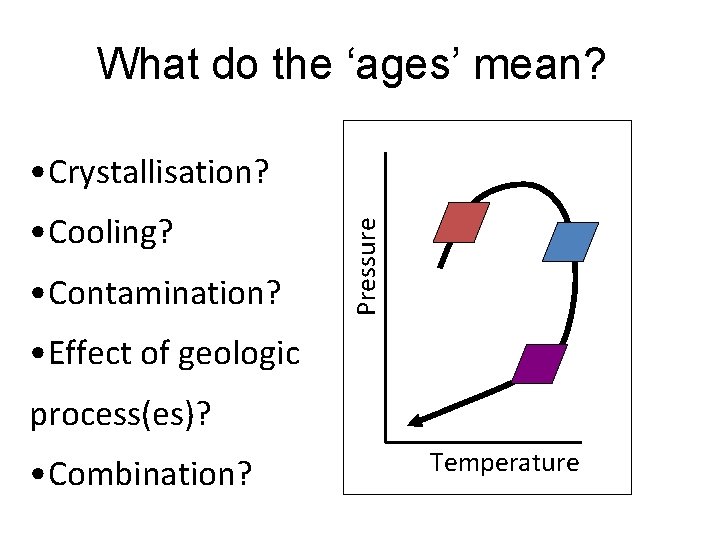
What do the ‘ages’ mean? • Cooling? • Contamination? Pressure • Crystallisation? • Effect of geologic process(es)? • Combination? Temperature
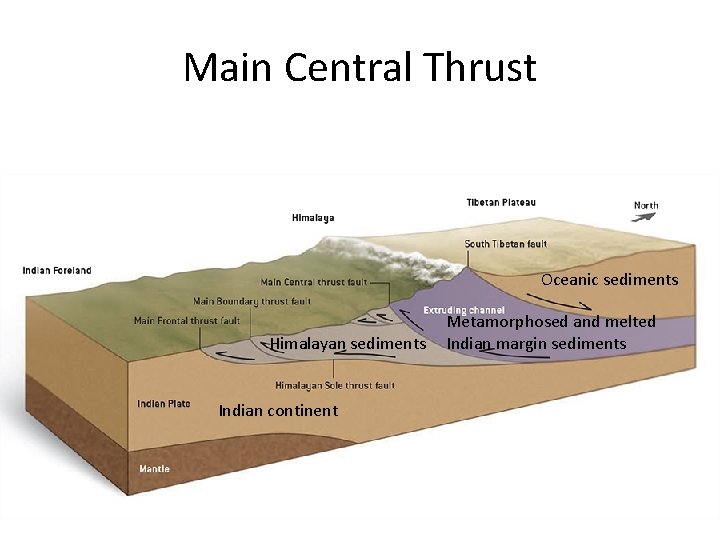
Main Central Thrust Oceanic sediments Himalayan sediments Indian continent Metamorphosed and melted Indian margin sediments
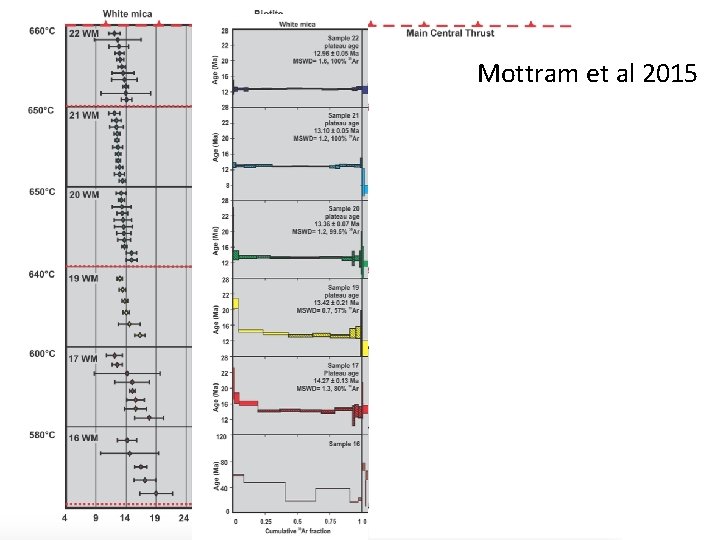
Mottram et al 2015
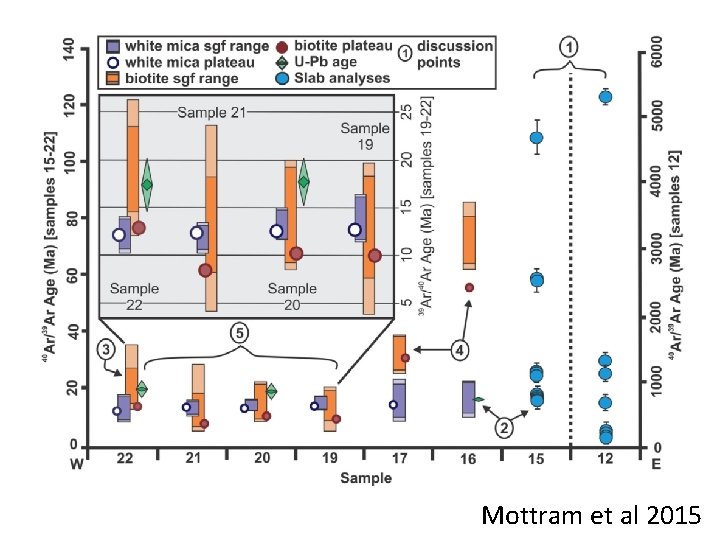
Mottram et al 2015

Geochronology basics What haven’t we covered: Intermediate daughter isotopes Initial disequilibrium Isotopic model ages Isochrons Fission track plus much more…

Take home messages • Geochronometers – Initial P/D ratios – Closed vs open system behaviour – Which mechanisms for alteration, disturbance, ‘scatter’ in data (outside of analytical) may be expected? – Closure (and formation) temperature
- Slides: 44