Introduction to Ferroelectric Materials and Devices 426415 1







- Slides: 71

Introduction to Ferroelectric Materials and Devices 426415 1

Objectives • To have basic knowledge of ferroelectric material • To understand piezoelectric effects • To describe its application • To understand lead zirconate titanate (PZT) solid solution system 2

What is this material ? 3

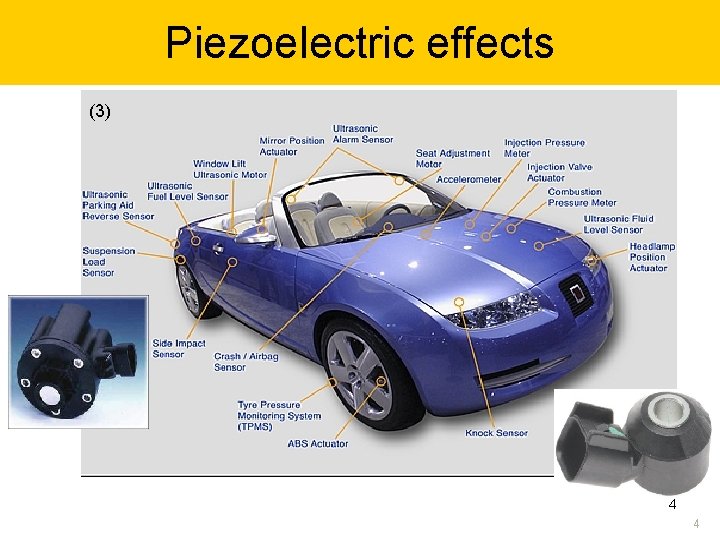
Piezoelectric effects (3) (1) (2) 4 4

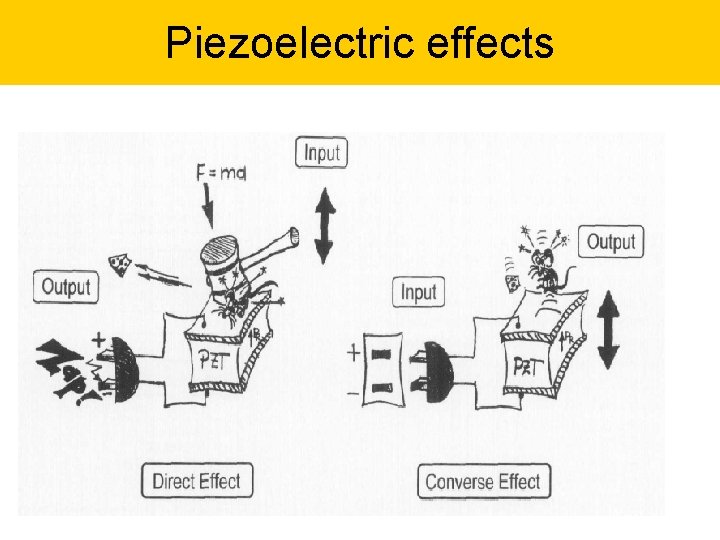
Piezoelectric effects Direct effect D = d. T + TE Converse effect S = s. ET + d. E D is dielectric displacement = polarization, T is the stress, E is the electric field, S = the strain, s = the material compliance (inverse of modulus of elasticity), = dielectric constant, d = piezoelectric (charge) constant 5

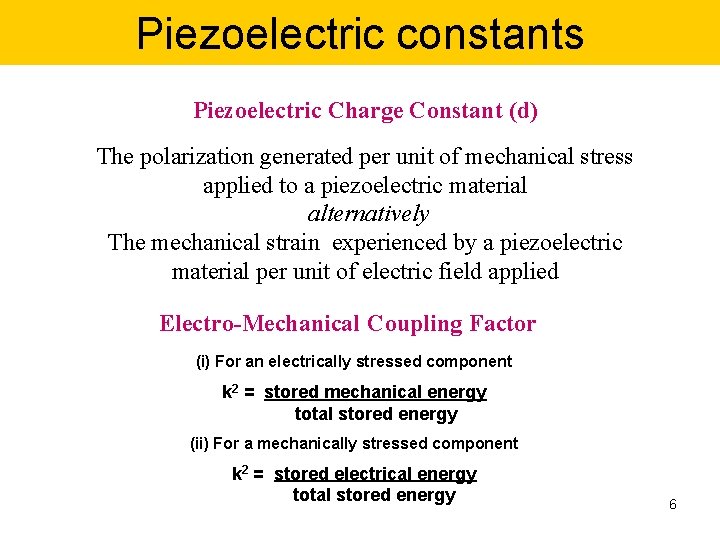
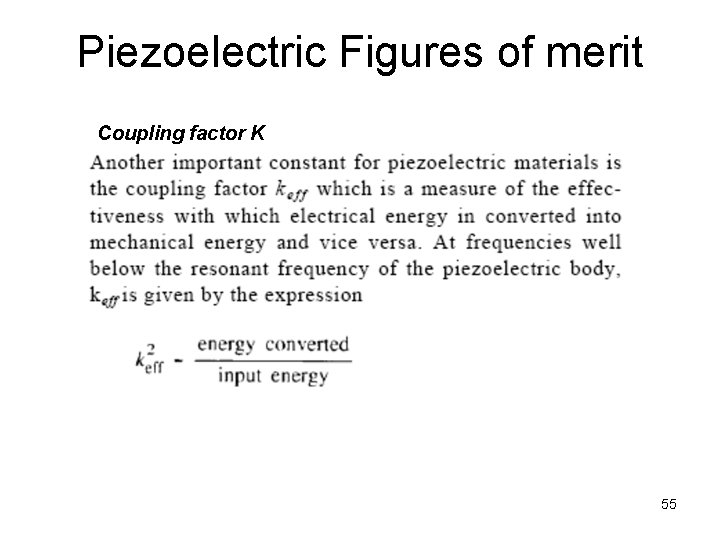
Piezoelectric constants Piezoelectric Charge Constant (d) The polarization generated per unit of mechanical stress applied to a piezoelectric material alternatively The mechanical strain experienced by a piezoelectric material per unit of electric field applied Electro-Mechanical Coupling Factor (i) For an electrically stressed component k 2 = stored mechanical energy total stored energy (ii) For a mechanically stressed component k 2 = stored electrical energy total stored energy 6

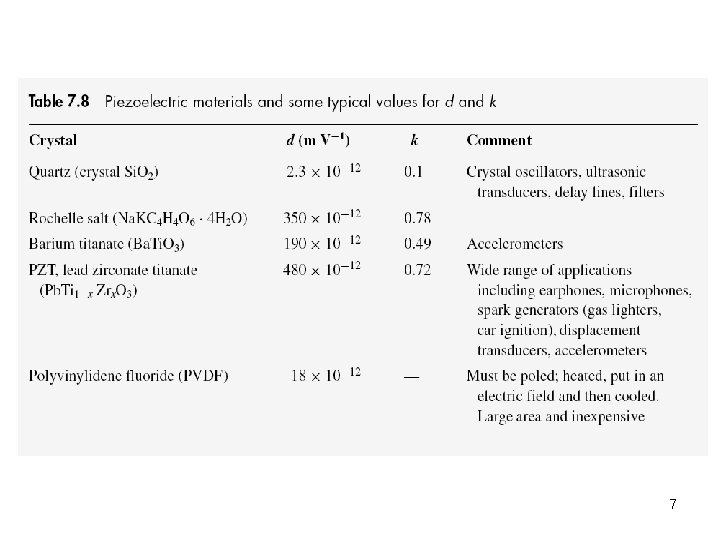
7

Polarization Dipole moment 5 Basic polarizations 8

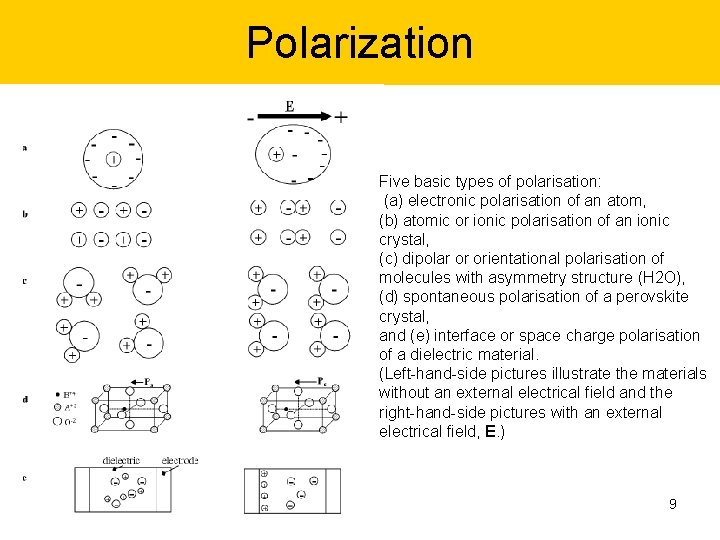
Polarization Five basic types of polarisation: (a) electronic polarisation of an atom, (b) atomic or ionic polarisation of an ionic crystal, (c) dipolar or orientational polarisation of molecules with asymmetry structure (H 2 O), (d) spontaneous polarisation of a perovskite crystal, and (e) interface or space charge polarisation of a dielectric material. (Left-hand-side pictures illustrate the materials without an external electrical field and the right-hand-side pictures with an external electrical field, E. ) 9

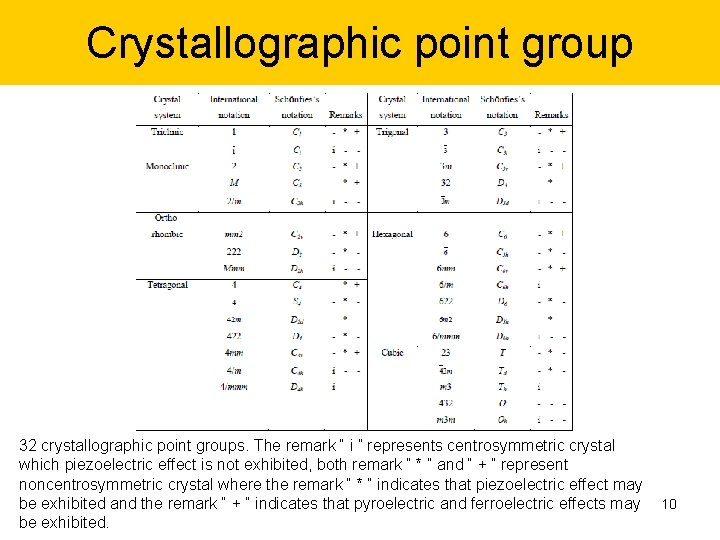
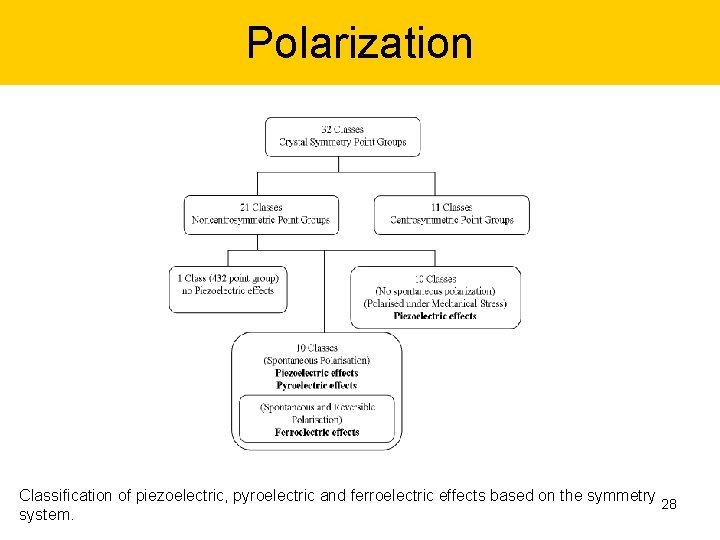
Crystallographic point group 32 crystallographic point groups. The remark “ i ” represents centrosymmetric crystal which piezoelectric effect is not exhibited, both remark “ * ” and “ + ” represent noncentrosymmetric crystal where the remark “ * ” indicates that piezoelectric effect may be exhibited and the remark “ + ” indicates that pyroelectric and ferroelectric effects may 10 be exhibited.

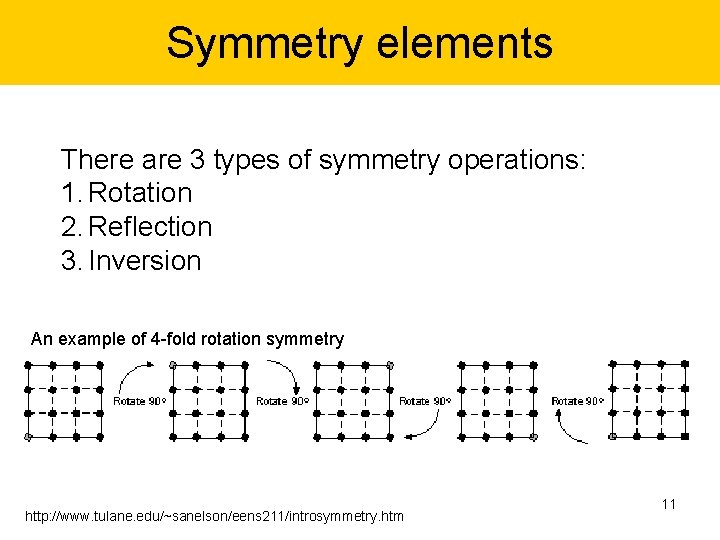
Symmetry elements There are 3 types of symmetry operations: 1. Rotation 2. Reflection 3. Inversion An example of 4 -fold rotation symmetry http: //www. tulane. edu/~sanelson/eens 211/introsymmetry. htm 11

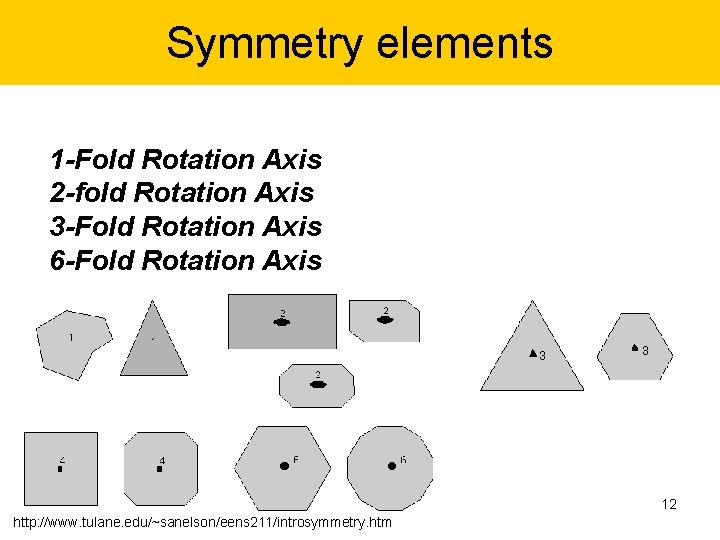
Symmetry elements 1 -Fold Rotation Axis 2 -fold Rotation Axis 3 -Fold Rotation Axis 6 -Fold Rotation Axis 12 http: //www. tulane. edu/~sanelson/eens 211/introsymmetry. htm

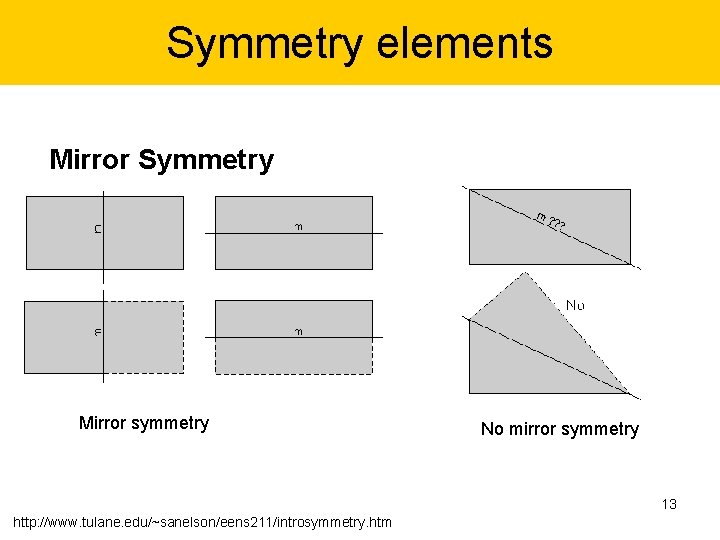
Symmetry elements Mirror Symmetry Mirror symmetry No mirror symmetry 13 http: //www. tulane. edu/~sanelson/eens 211/introsymmetry. htm

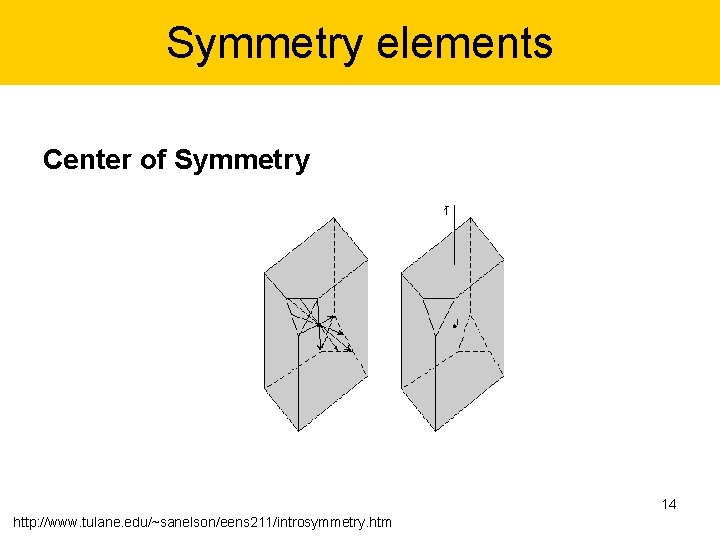
Symmetry elements Center of Symmetry 14 http: //www. tulane. edu/~sanelson/eens 211/introsymmetry. htm

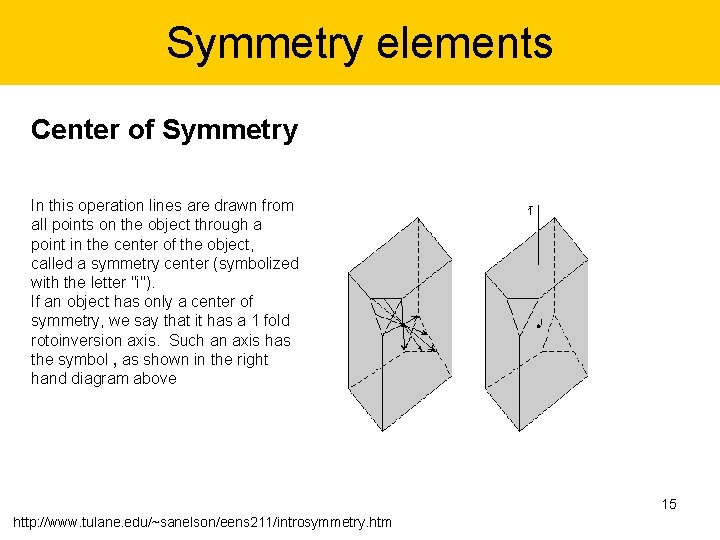
Symmetry elements Center of Symmetry In this operation lines are drawn from all points on the object through a point in the center of the object, called a symmetry center (symbolized with the letter "i"). If an object has only a center of symmetry, we say that it has a 1 fold rotoinversion axis. Such an axis has the symbol , as shown in the right hand diagram above 15 http: //www. tulane. edu/~sanelson/eens 211/introsymmetry. htm

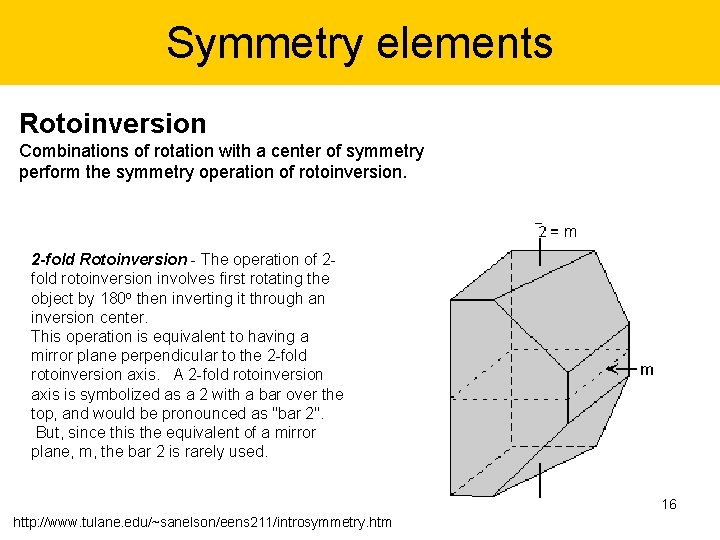
Symmetry elements Rotoinversion Combinations of rotation with a center of symmetry perform the symmetry operation of rotoinversion. 2 -fold Rotoinversion - The operation of 2 fold rotoinversion involves first rotating the object by 180 o then inverting it through an inversion center. This operation is equivalent to having a mirror plane perpendicular to the 2 -fold rotoinversion axis. A 2 -fold rotoinversion axis is symbolized as a 2 with a bar over the top, and would be pronounced as "bar 2". But, since this the equivalent of a mirror plane, m, the bar 2 is rarely used. 16 http: //www. tulane. edu/~sanelson/eens 211/introsymmetry. htm

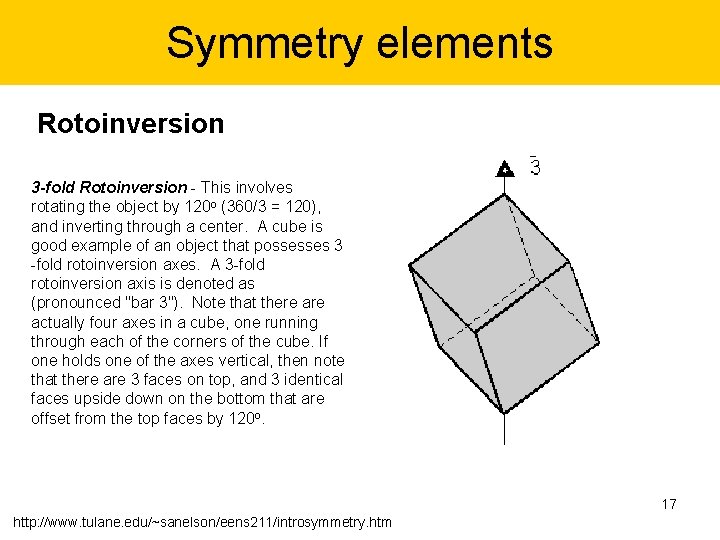
Symmetry elements Rotoinversion 3 -fold Rotoinversion - This involves rotating the object by 120 o (360/3 = 120), and inverting through a center. A cube is good example of an object that possesses 3 -fold rotoinversion axes. A 3 -fold rotoinversion axis is denoted as (pronounced "bar 3"). Note that there actually four axes in a cube, one running through each of the corners of the cube. If one holds one of the axes vertical, then note that there are 3 faces on top, and 3 identical faces upside down on the bottom that are offset from the top faces by 120 o. 17 http: //www. tulane. edu/~sanelson/eens 211/introsymmetry. htm

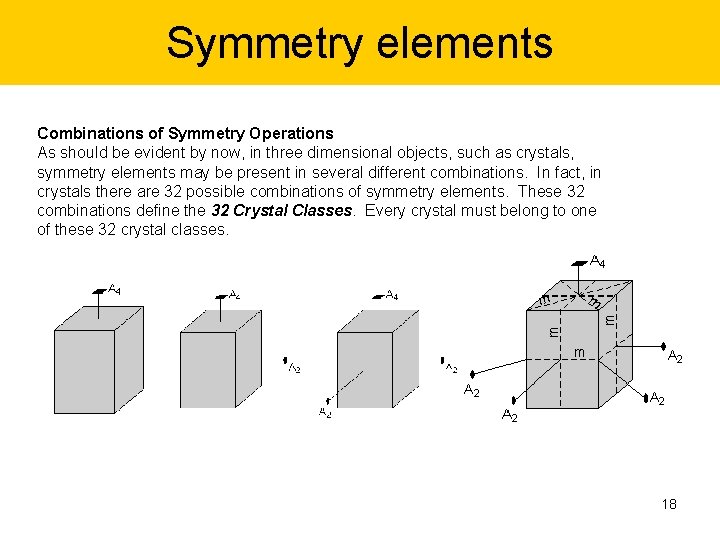
Symmetry elements Combinations of Symmetry Operations As should be evident by now, in three dimensional objects, such as crystals, symmetry elements may be present in several different combinations. In fact, in crystals there are 32 possible combinations of symmetry elements. These 32 combinations define the 32 Crystal Classes. Every crystal must belong to one of these 32 crystal classes. 18

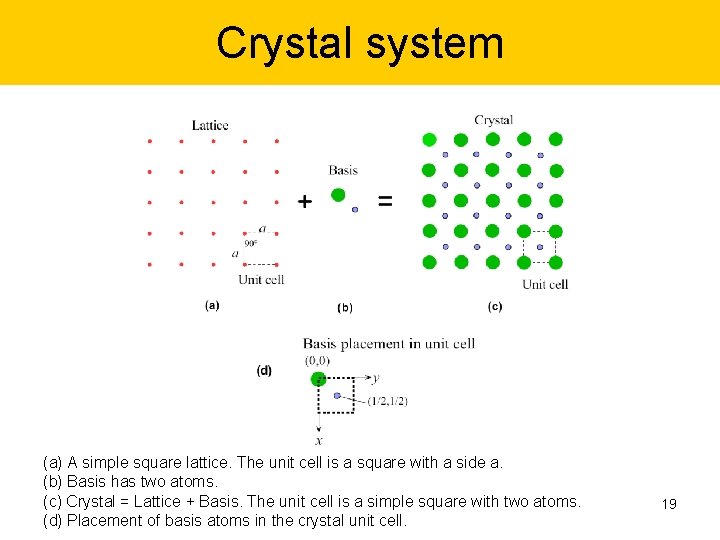
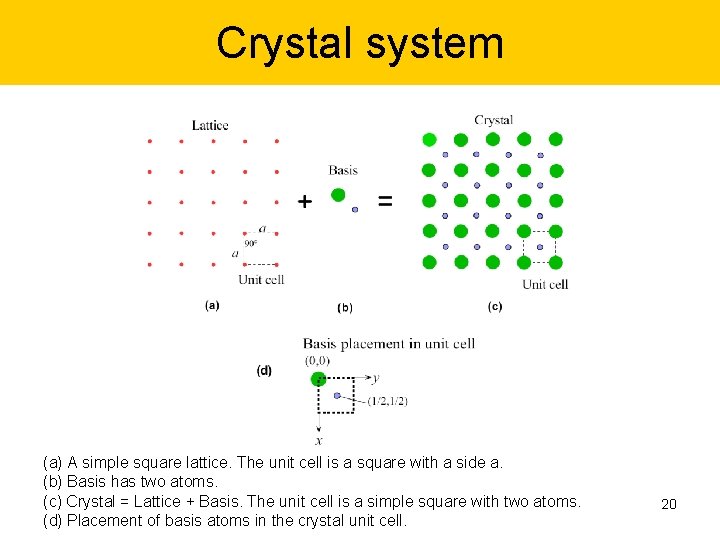
Crystal system (a) A simple square lattice. The unit cell is a square with a side a. (b) Basis has two atoms. (c) Crystal = Lattice + Basis. The unit cell is a simple square with two atoms. (d) Placement of basis atoms in the crystal unit cell. 19

Crystal system (a) A simple square lattice. The unit cell is a square with a side a. (b) Basis has two atoms. (c) Crystal = Lattice + Basis. The unit cell is a simple square with two atoms. (d) Placement of basis atoms in the crystal unit cell. 20

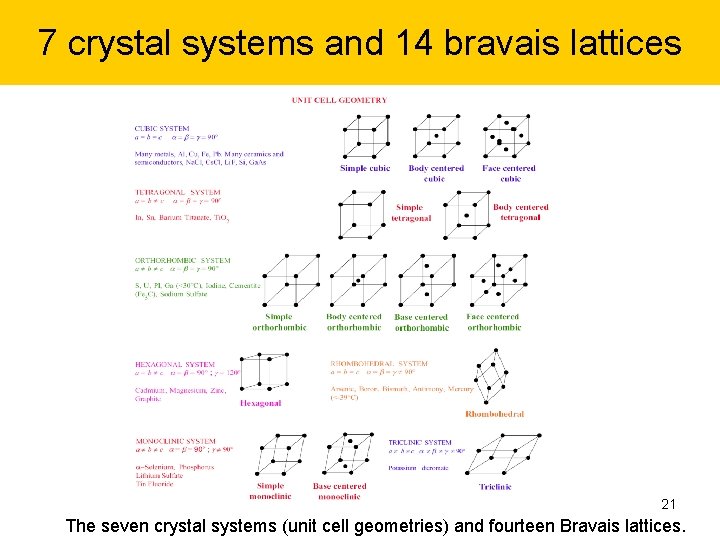
7 crystal systems and 14 bravais lattices 21 The seven crystal systems (unit cell geometries) and fourteen Bravais lattices.

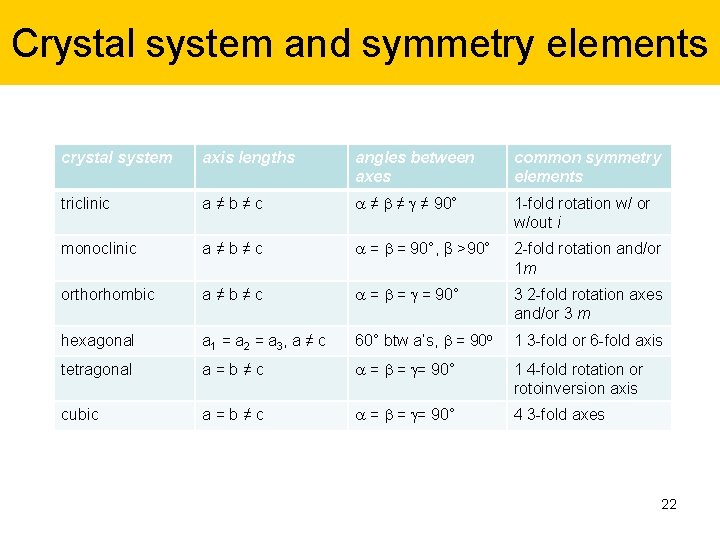
Crystal system and symmetry elements crystal system axis lengths angles between axes common symmetry elements triclinic a ≠ b ≠ c ≠ ≠ ≠ 90° 1 -fold rotation w/ or w/out i monoclinic a ≠ b ≠ c = = 90°, β >90° 2 -fold rotation and/or 1 m orthorhombic a ≠ b ≠ c = = = 90° 3 2 -fold rotation axes and/or 3 m hexagonal a 1 = a 2 = a 3, a ≠ c 60° btw a’s, = 90 o 1 3 -fold or 6 -fold axis tetragonal a = b ≠ c = = = 90° 1 4 -fold rotation or rotoinversion axis cubic a = b ≠ c = = = 90° 4 3 -fold axes 22

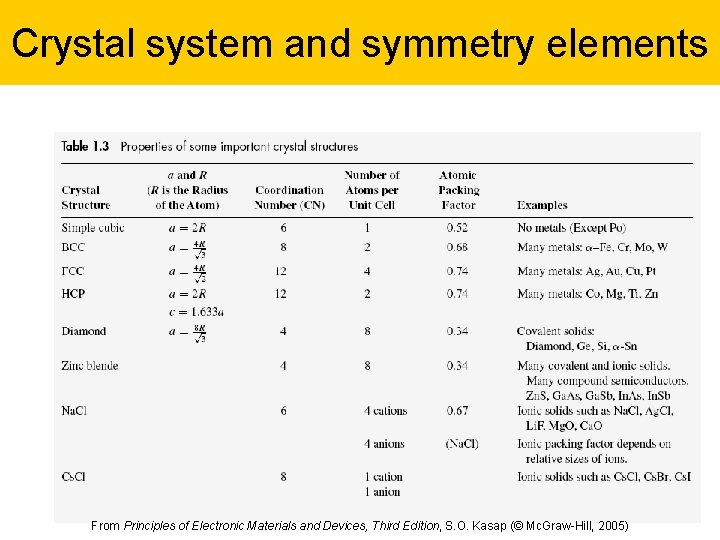
Crystal system and symmetry elements 23 From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw-Hill, 2005)

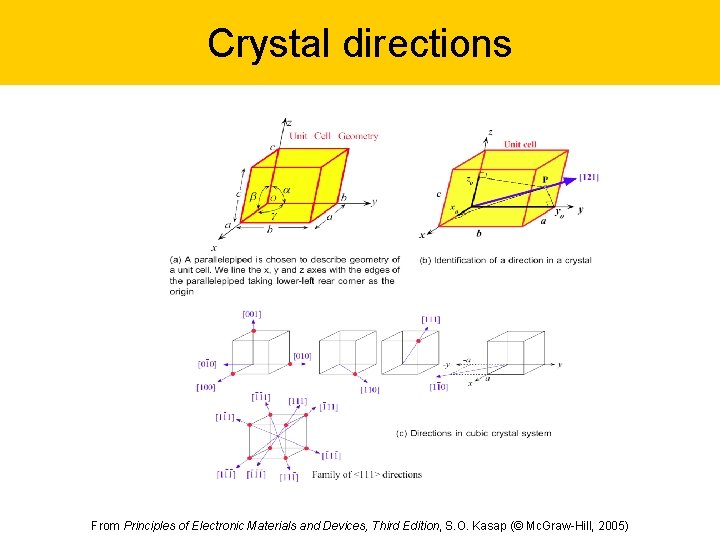
Crystal directions From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw-Hill, 2005)

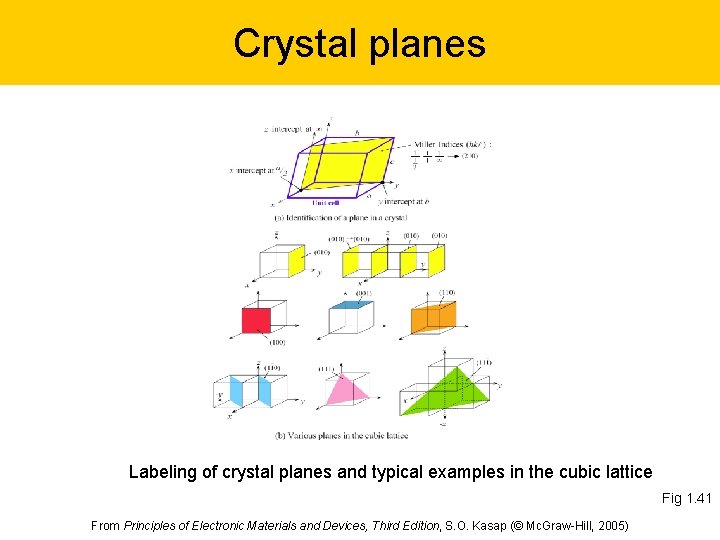
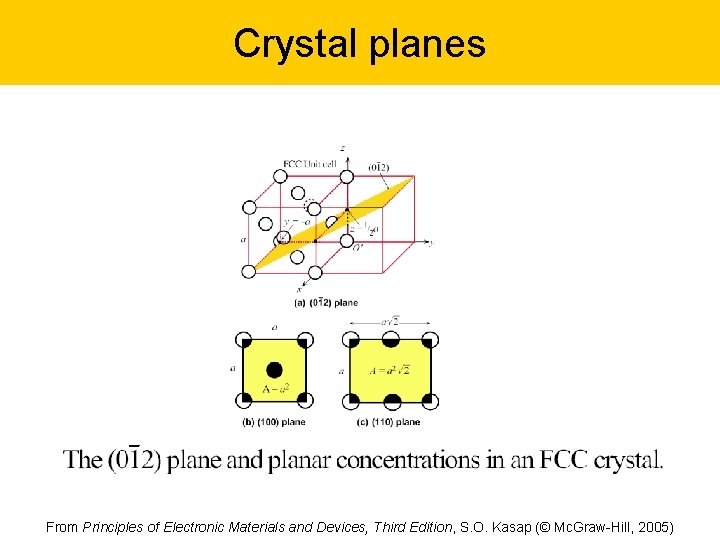
Crystal planes Labeling of crystal planes and typical examples in the cubic lattice Fig 1. 41 From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw-Hill, 2005)

Crystal planes From Principles of Electronic Materials and Devices, Third Edition, S. O. Kasap (© Mc. Graw-Hill, 2005)

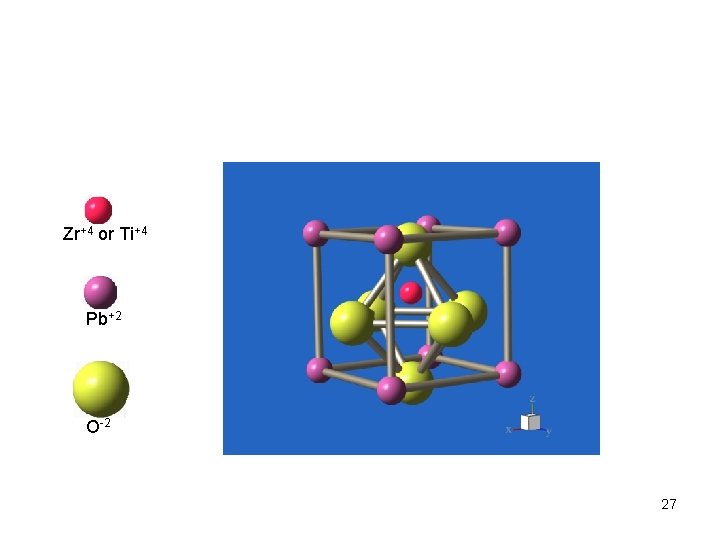
Zr+4 or Ti+4 Pb+2 O-2 27

Polarization Classification of piezoelectric, pyroelectric and ferroelectric effects based on the symmetry 28 system.

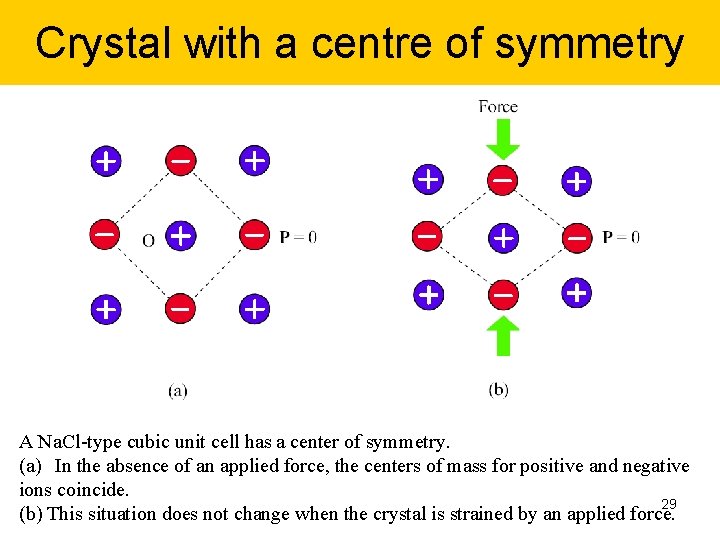
Crystal with a centre of symmetry A Na. Cl-type cubic unit cell has a center of symmetry. (a) In the absence of an applied force, the centers of mass for positive and negative ions coincide. 29 (b) This situation does not change when the crystal is strained by an applied force.

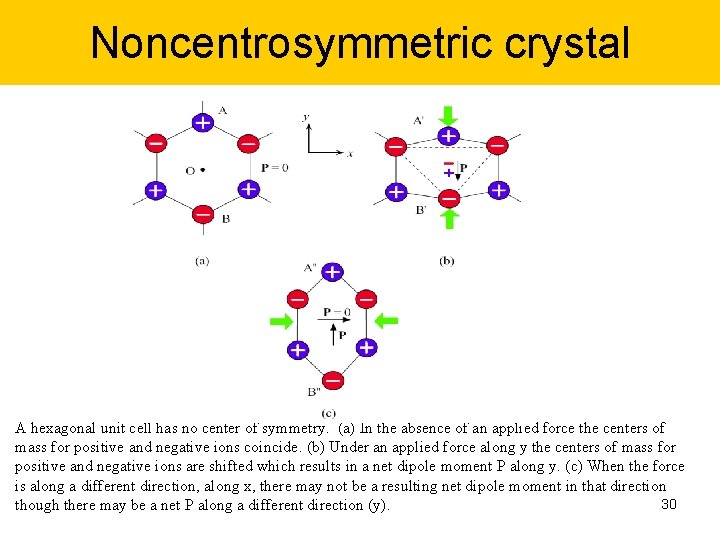
Noncentrosymmetric crystal A hexagonal unit cell has no center of symmetry. (a) In the absence of an applied force the centers of mass for positive and negative ions coincide. (b) Under an applied force along y the centers of mass for positive and negative ions are shifted which results in a net dipole moment P along y. (c) When the force is along a different direction, along x, there may not be a resulting net dipole moment in that direction 30 though there may be a net P along a different direction (y).

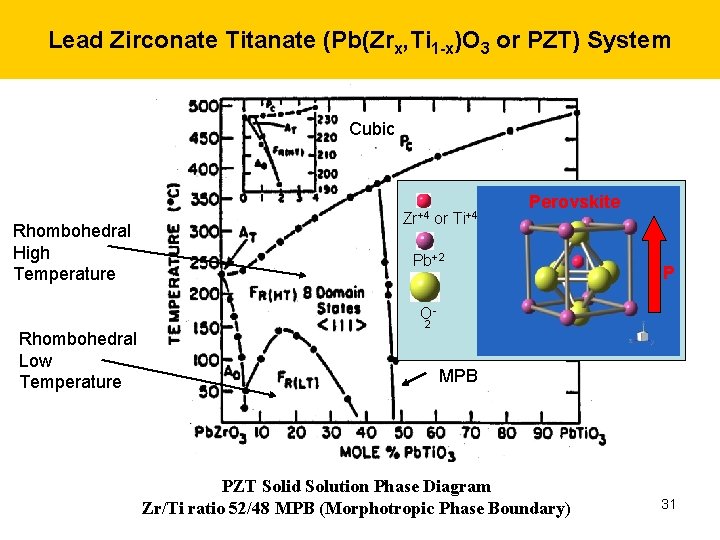
Lead Zirconate Titanate (Pb(Zrx, Ti 1 -x)O 3 or PZT) System Cubic Rhombohedral High Temperature Zr+4 or Ti+4 Perovskite Tetragonal Pb+2 P O- Rhombohedral Low Temperature 2 MPB PZT Solid Solution Phase Diagram Zr/Ti ratio 52/48 MPB (Morphotropic Phase Boundary) 31

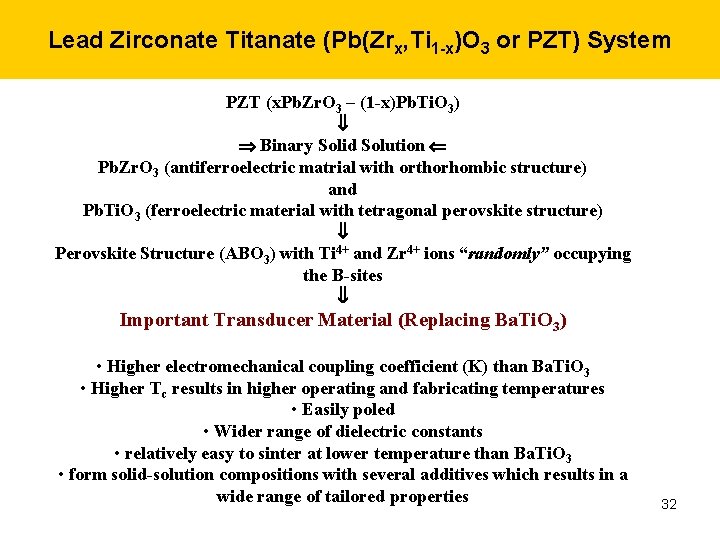
Lead Zirconate Titanate (Pb(Zrx, Ti 1 -x)O 3 or PZT) System PZT (x. Pb. Zr. O 3 – (1 -x)Pb. Ti. O 3) Binary Solid Solution Pb. Zr. O 3 (antiferroelectric matrial with orthorhombic structure) and Pb. Ti. O 3 (ferroelectric material with tetragonal perovskite structure) Perovskite Structure (ABO 3) with Ti 4+ and Zr 4+ ions “randomly” occupying the B-sites Important Transducer Material (Replacing Ba. Ti. O 3) • Higher electromechanical coupling coefficient (K) than Ba. Ti. O 3 • Higher Tc results in higher operating and fabricating temperatures • Easily poled • Wider range of dielectric constants • relatively easy to sinter at lower temperature than Ba. Ti. O 3 • form solid-solution compositions with several additives which results in a wide range of tailored properties 32

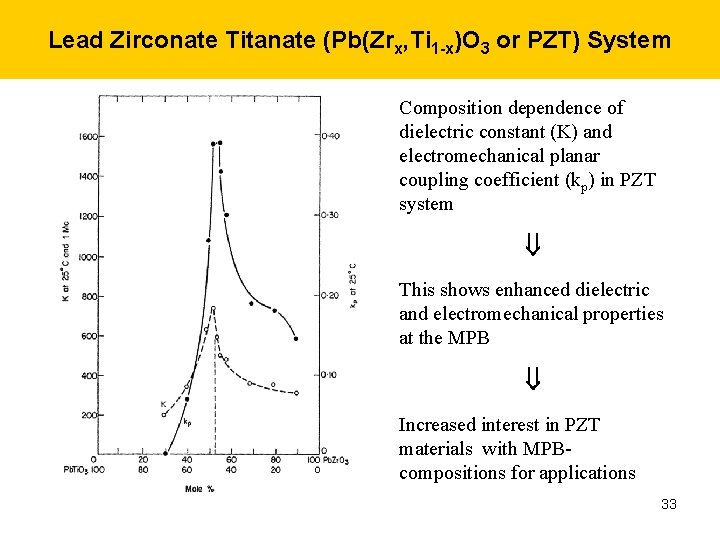
Lead Zirconate Titanate (Pb(Zrx, Ti 1 -x)O 3 or PZT) System Composition dependence of dielectric constant (K) and electromechanical planar coupling coefficient (kp) in PZT system This shows enhanced dielectric and electromechanical properties at the MPB Increased interest in PZT materials with MPBcompositions for applications 33

Lead Zirconate Titanate (Pb(Zrx, Ti 1 -x)O 3 or PZT) System Advantages of PZT Solid-Solution System • High Tc across the diagram leads to more stable ferroelectric states over wide temperature ranges • There is a two-phase region near the Morphotropic Phase Boundary (MPB) (52/48 Zr/Ti composition) separating rhombohedral (with 8 domain states) and tetragonal (with 6 domain states) phases • In the two-phase region, the poling may draw upon 14 orientation states leading to exceptional polability • Near vertical MPB results in property enhancement over wider temperature range for chosen compositions near the MPB 34

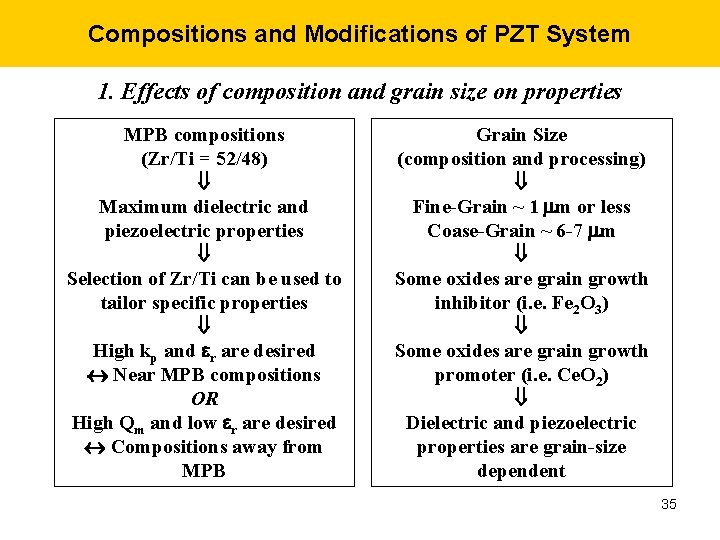
Compositions and Modifications of PZT System 1. Effects of composition and grain size on properties MPB compositions (Zr/Ti = 52/48) Maximum dielectric and piezoelectric properties Selection of Zr/Ti can be used to tailor specific properties High kp and r are desired Near MPB compositions OR High Qm and low r are desired Compositions away from MPB Grain Size (composition and processing) Fine-Grain ~ 1 mm or less Coase-Grain ~ 6 -7 mm Some oxides are grain growth inhibitor (i. e. Fe 2 O 3) Some oxides are grain growth promoter (i. e. Ce. O 2) Dielectric and piezoelectric properties are grain-size dependent 35

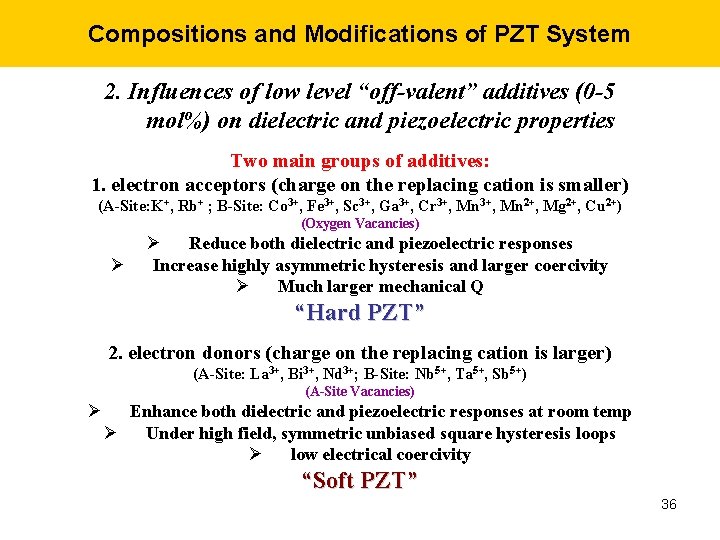
Compositions and Modifications of PZT System 2. Influences of low level “off-valent” additives (0 -5 mol%) on dielectric and piezoelectric properties Two main groups of additives: 1. electron acceptors (charge on the replacing cation is smaller) (A-Site: K+, Rb+ ; B-Site: Co 3+, Fe 3+, Sc 3+, Ga 3+, Cr 3+, Mn 2+, Mg 2+, Cu 2+) (Oxygen Vacancies) Ø Ø Reduce both dielectric and piezoelectric responses Increase highly asymmetric hysteresis and larger coercivity Ø Much larger mechanical Q “Hard PZT” 2. electron donors (charge on the replacing cation is larger) (A-Site: La 3+, Bi 3+, Nd 3+; B-Site: Nb 5+, Ta 5+, Sb 5+) (A-Site Vacancies) Ø Enhance both dielectric and piezoelectric responses at room temp Ø Under high field, symmetric unbiased square hysteresis loops Ø low electrical coercivity “Soft PZT” 36

Conclusion Piezoelectric effects 1. mechanical energy electrical energy : sensors 2. electrical energy mechanical energy : actuators 2. 3. noncentrosymmetric crystal, perovskite structure Lead Zirconate Titanate Pb(Zrx, Ti 1 -x)O 3 PZT near MPB high piezoelectric response (high K and d) Hard PZT additive = electron acceptors (A-Site: K+, Rb+ ; B-Site: Co 3+, Fe 3+, Sc 3+, Ga 3+, Cr 3+, Mn 3+, Mn 2+, Mg 2+, Cu 2+) low piezoelectric response Soft PZT additive = electron donors (A-Site: La 3+, Bi 3+, Nd 3+; B-Site: Nb 5+, Ta 5+, Sb 5+) high piezoelectric response 37

Modified PZT System “Hard PZT” Materials § Curie temperature above 300 C § NOT easily poled or depoled except at high temperature § Small piezoelectric d constants § Good linearity and low hysteresis § High mechanical Q values § Withstand high loads and voltages “Soft PZT” Materials § Lower Curie temperature § Readily poled or depoled at room temperature with high field § Large piezoelectric d constants § Poor linearity and highly hysteretic § Large dielectric constants and dissipation factors § Limited uses at high field and high frequency 38

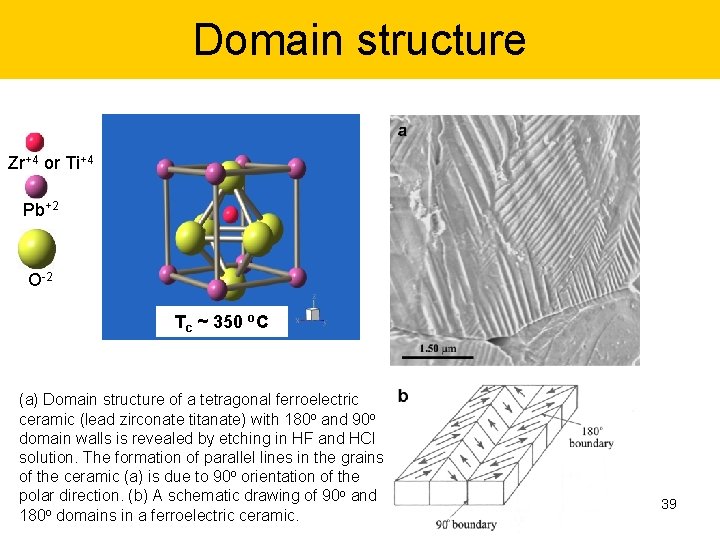
Domain structure Zr+4 or Ti+4 Pb+2 O-2 Tc ~ 350 o. C (a) Domain structure of a tetragonal ferroelectric ceramic (lead zirconate titanate) with 180 o and 90 o domain walls is revealed by etching in HF and HCl solution. The formation of parallel lines in the grains of the ceramic (a) is due to 90 o orientation of the polar direction. (b) A schematic drawing of 90 o and 180 o domains in a ferroelectric ceramic. 39

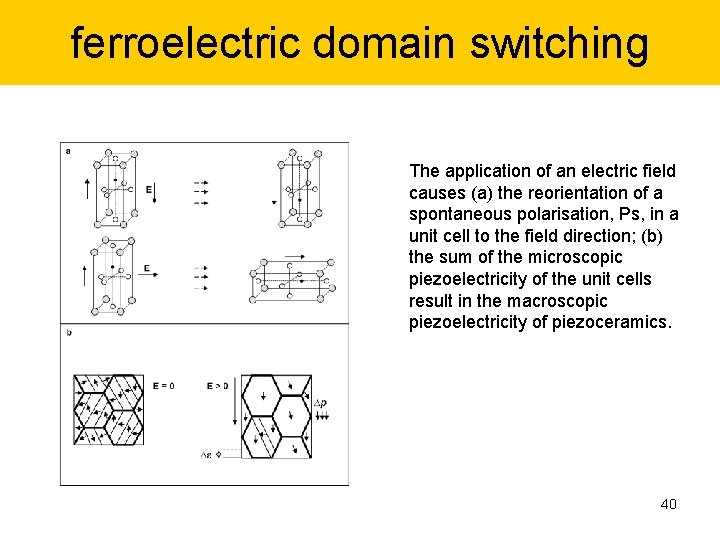
ferroelectric domain switching The application of an electric field causes (a) the reorientation of a spontaneous polarisation, Ps, in a unit cell to the field direction; (b) the sum of the microscopic piezoelectricity of the unit cells result in the macroscopic piezoelectricity of piezoceramics. 40

Compositions and Modifications of PZT System 2. Modification by element substitution Element substitution cations in perovskite lattice (Pb 2+, Ti 4+, and Zr 4+) are replaced partially by other cations with the same chemical valence and similar ionic radii and solid solution is formed Pb 2+ substituted by alkali-earth metals, Mg 2+, Ca 2+, Sr 2+, and Ba 2+ PZT replaced partially by Ca 2+or Sr 2+ Tc BUT kp, 33 , and d 31 ¨Shift of MPB towards the Zr-rich side ¨ Density due to fluxing effect of Ca or Sr ions Ti 4+ and Zr 4+ substituted by Sn 4+ and Hf 4+ , respectively Ti 4+ replaced partially by Sn 4+ c/a ratio decreases with increasing Sn 4+ content Tc and stability of kp and 33 41

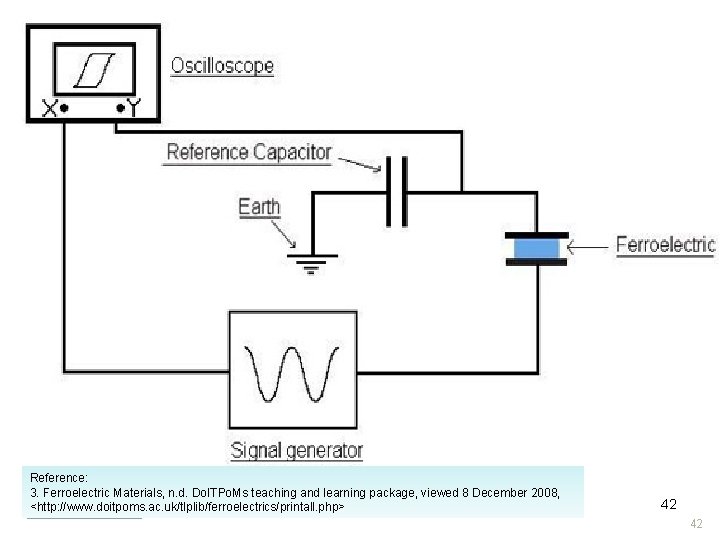
Electrode (3) Electrode Polarization Electrode Electric Field Electrode Reference: 3. Ferroelectric Materials, n. d. Do. ITPo. Ms teaching and learning package, viewed 8 December 2008, <http: //www. doitpoms. ac. uk/tlplib/ferroelectrics/printall. php> Electrode 42 42

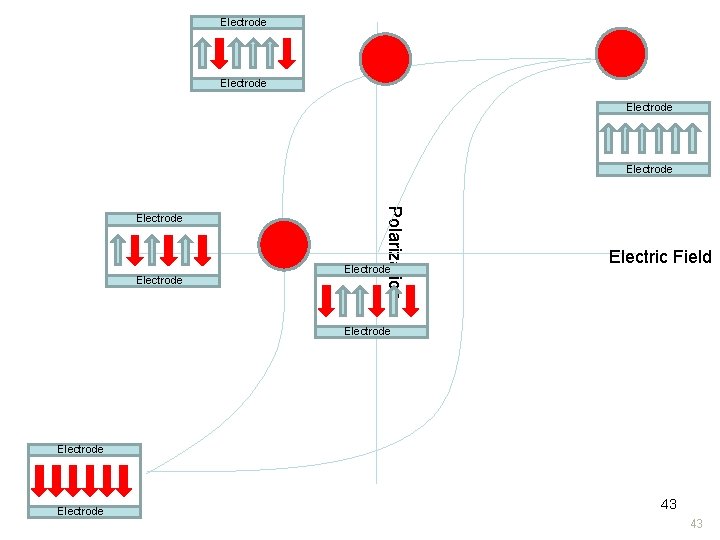
Electrode Electrode Polarization Electrode Electric Field Electrode 43 43

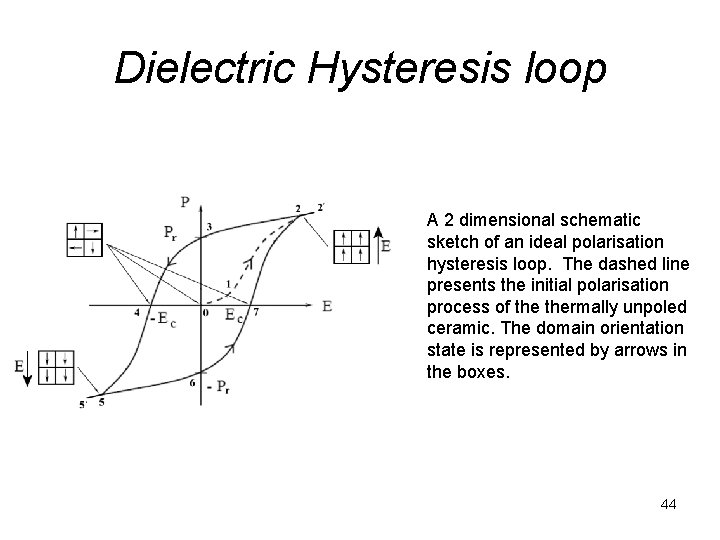
Dielectric Hysteresis loop A 2 dimensional schematic sketch of an ideal polarisation hysteresis loop. The dashed line presents the initial polarisation process of thermally unpoled ceramic. The domain orientation state is represented by arrows in the boxes. 44

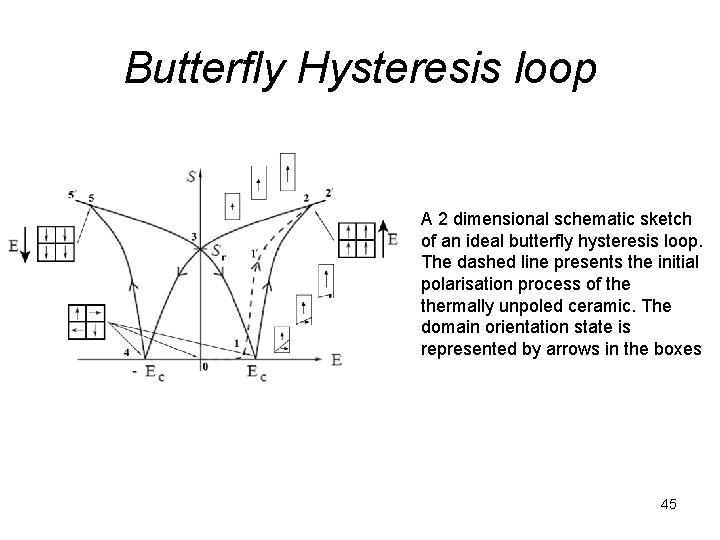
Butterfly Hysteresis loop A 2 dimensional schematic sketch of an ideal butterfly hysteresis loop. The dashed line presents the initial polarisation process of thermally unpoled ceramic. The domain orientation state is represented by arrows in the boxes 45

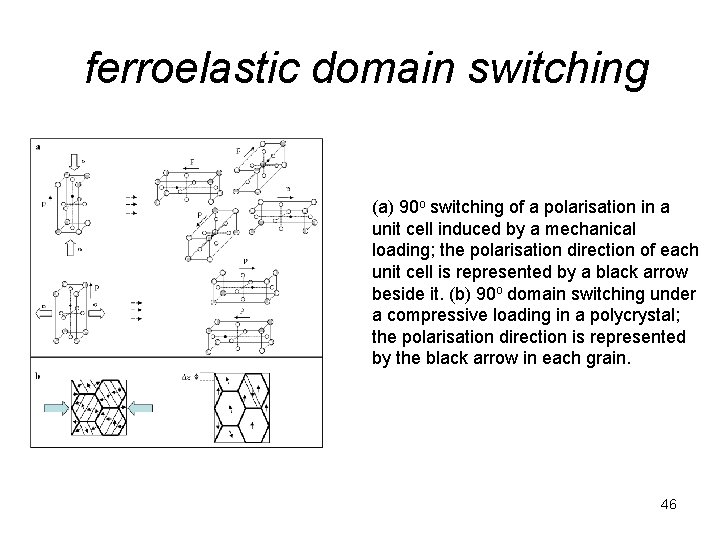
ferroelastic domain switching (a) 90 o switching of a polarisation in a unit cell induced by a mechanical loading; the polarisation direction of each unit cell is represented by a black arrow beside it. (b) 90 o domain switching under a compressive loading in a polycrystal; the polarisation direction is represented by the black arrow in each grain. 46

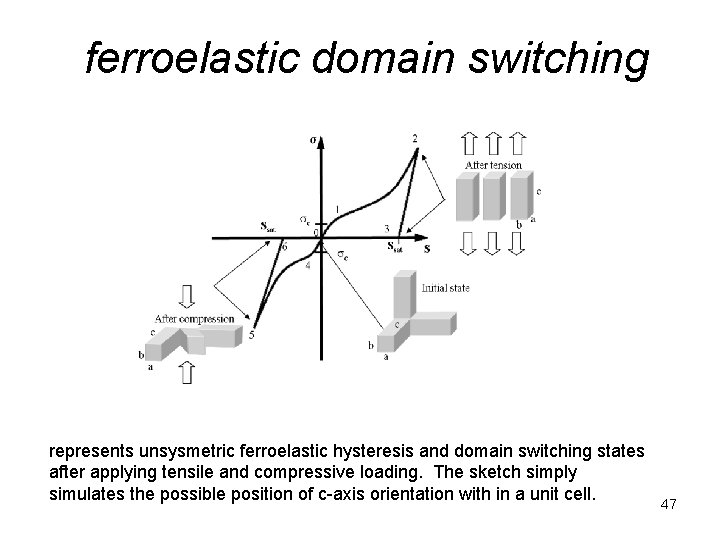
ferroelastic domain switching represents unsysmetric ferroelastic hysteresis and domain switching states after applying tensile and compressive loading. The sketch simply simulates the possible position of c-axis orientation with in a unit cell. 47

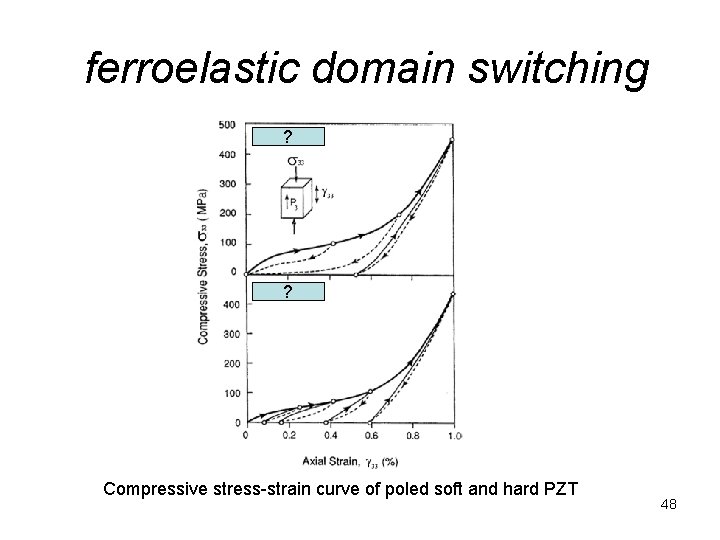
ferroelastic domain switching ? ? Compressive stress-strain curve of poled soft and hard PZT 48

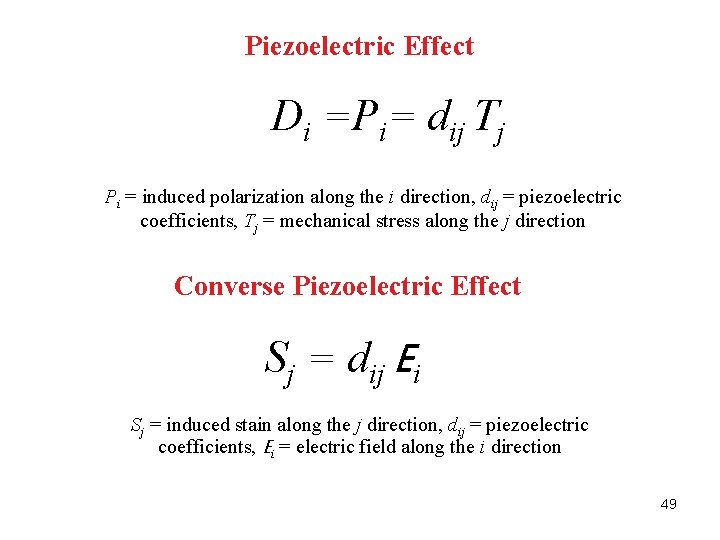
Piezoelectric Effect Di =Pi= dij Tj Pi = induced polarization along the i direction, dij = piezoelectric coefficients, Tj = mechanical stress along the j direction Converse Piezoelectric Effect Sj = dij Ei Sj = induced stain along the j direction, dij = piezoelectric coefficients, Ei = electric field along the i direction 49

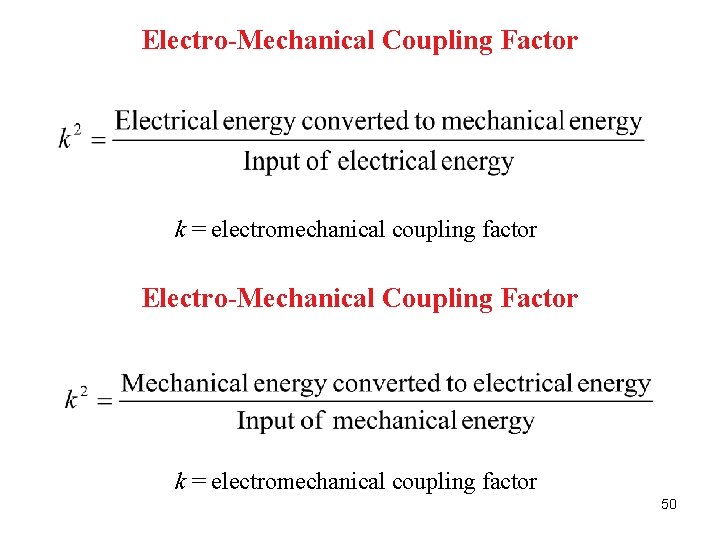
Electro-Mechanical Coupling Factor k = electromechanical coupling factor 50

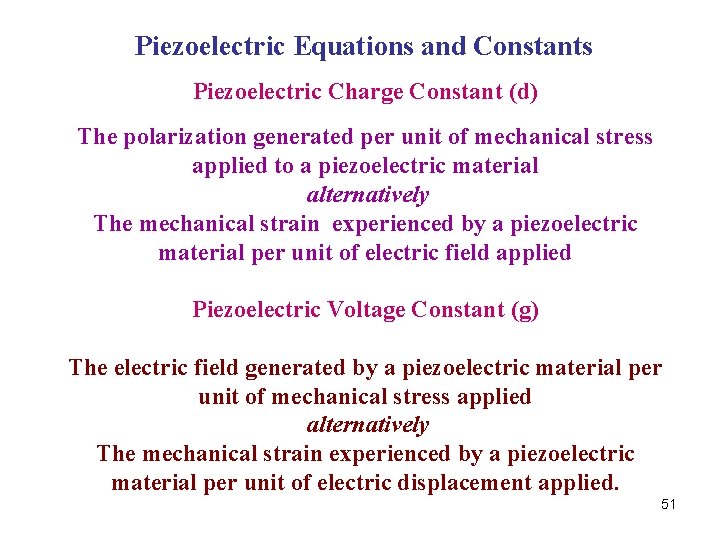
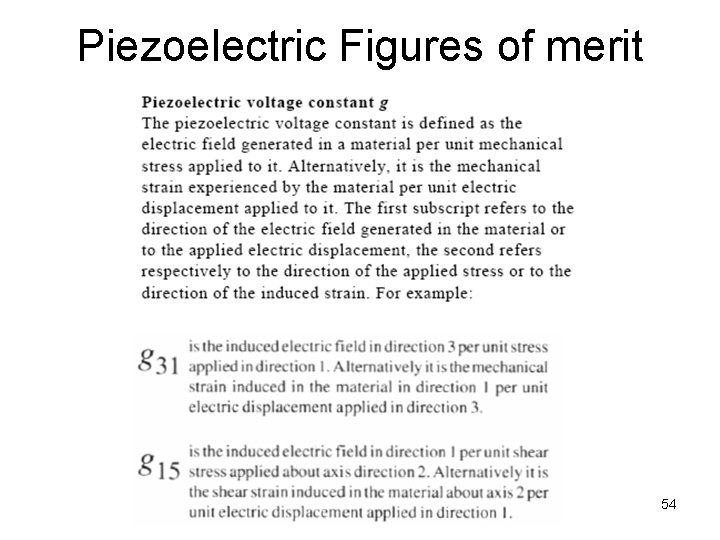
Piezoelectric Equations and Constants Piezoelectric Charge Constant (d) The polarization generated per unit of mechanical stress applied to a piezoelectric material alternatively The mechanical strain experienced by a piezoelectric material per unit of electric field applied Piezoelectric Voltage Constant (g) The electric field generated by a piezoelectric material per unit of mechanical stress applied alternatively The mechanical strain experienced by a piezoelectric material per unit of electric displacement applied. 51

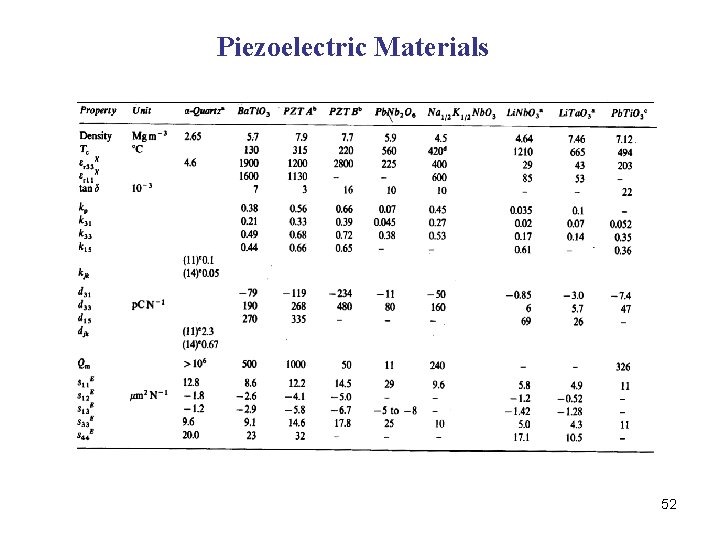
Piezoelectric Materials 52

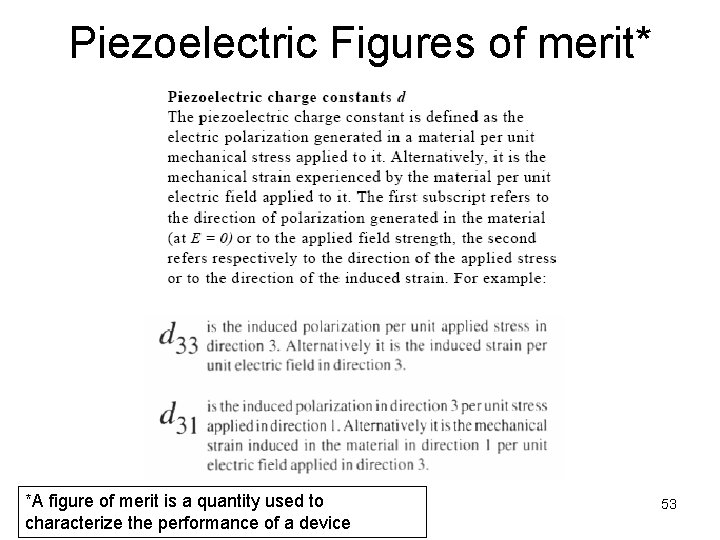
Piezoelectric Figures of merit* *A figure of merit is a quantity used to characterize the performance of a device 53

Piezoelectric Figures of merit 54

Piezoelectric Figures of merit Coupling factor K 55

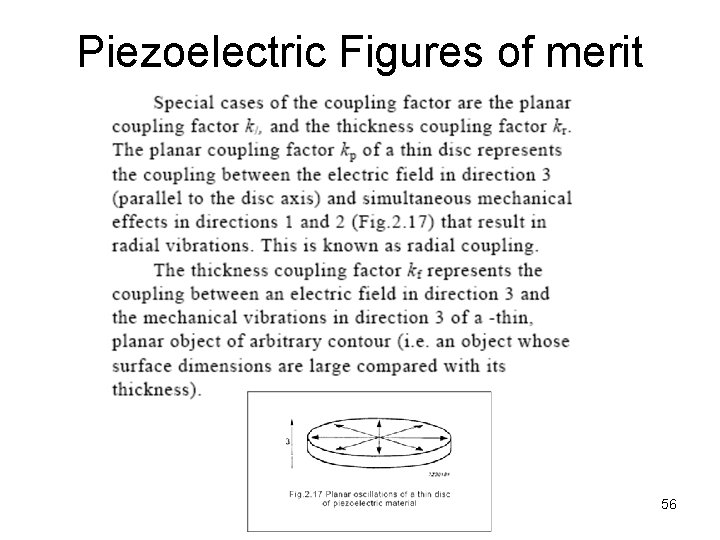
Piezoelectric Figures of merit 56

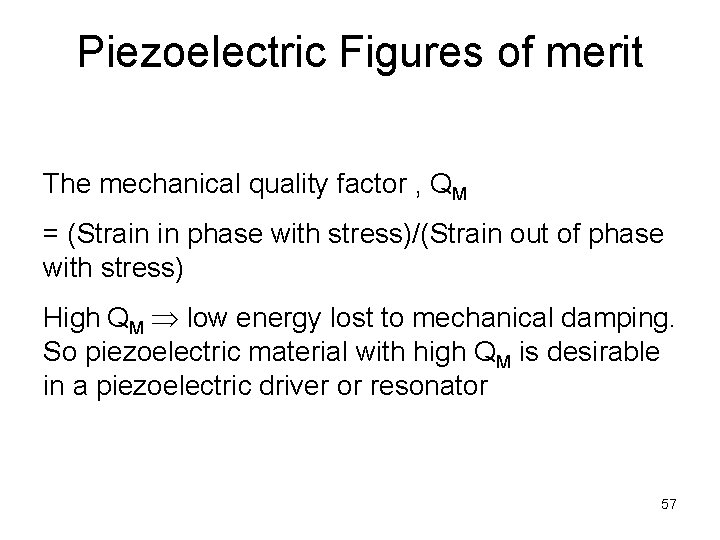
Piezoelectric Figures of merit The mechanical quality factor , QM = (Strain in phase with stress)/(Strain out of phase with stress) High QM low energy lost to mechanical damping. So piezoelectric material with high QM is desirable in a piezoelectric driver or resonator 57

Piezoelectric constants Permittivity 58

Piezoelectric constants 59

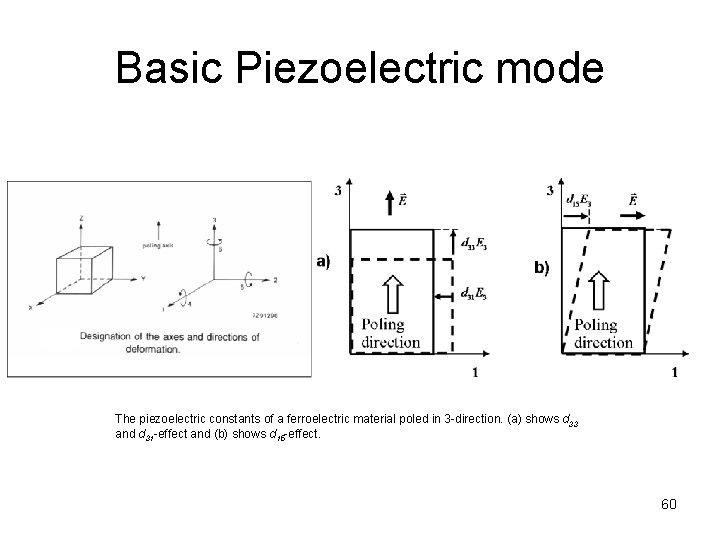
Basic Piezoelectric mode The piezoelectric constants of a ferroelectric material poled in 3 -direction. (a) shows d 33 and d 31 -effect and (b) shows d 15 -effect. 60

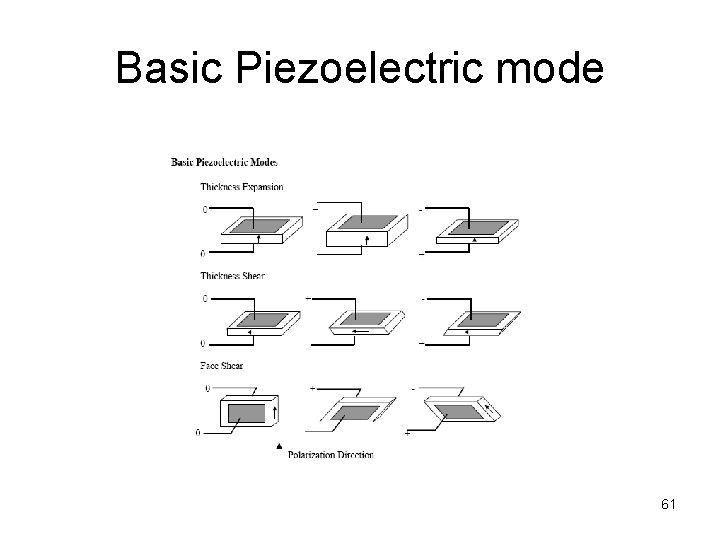
Basic Piezoelectric mode 61

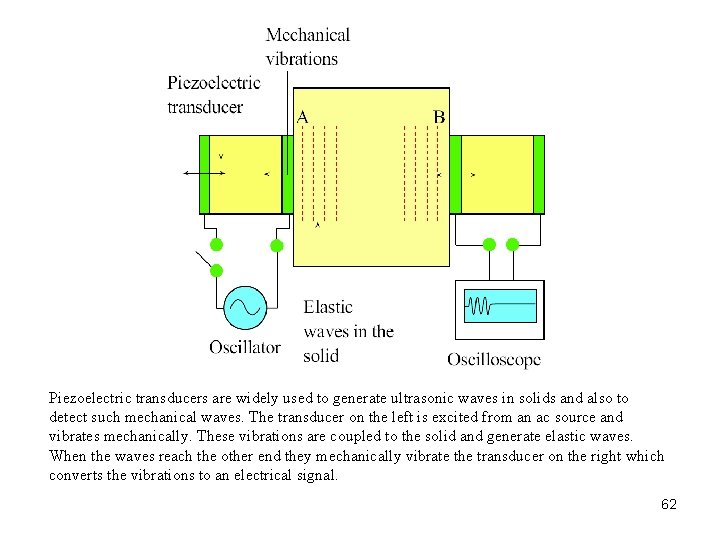
Piezoelectric transducers are widely used to generate ultrasonic waves in solids and also to detect such mechanical waves. The transducer on the left is excited from an ac source and vibrates mechanically. These vibrations are coupled to the solid and generate elastic waves. When the waves reach the other end they mechanically vibrate the transducer on the right which converts the vibrations to an electrical signal. 62

Piezoelectric Voltage Coefficient E = g. T E = electric field, g = piezoelectric voltage coefficient T = applied stress g = d/( o r) 63

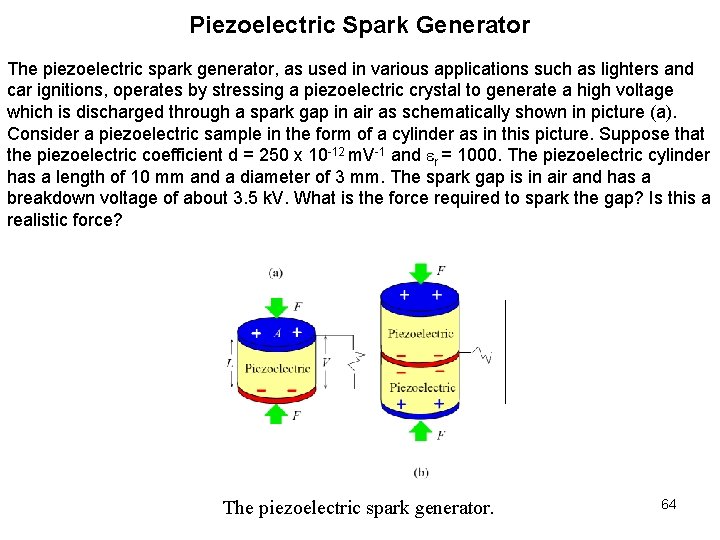
Piezoelectric Spark Generator The piezoelectric spark generator, as used in various applications such as lighters and car ignitions, operates by stressing a piezoelectric crystal to generate a high voltage which is discharged through a spark gap in air as schematically shown in picture (a). Consider a piezoelectric sample in the form of a cylinder as in this picture. Suppose that the piezoelectric coefficient d = 250 x 10 -12 m. V-1 and r = 1000. The piezoelectric cylinder has a length of 10 mm and a diameter of 3 mm. The spark gap is in air and has a breakdown voltage of about 3. 5 k. V. What is the force required to spark the gap? Is this a realistic force? The piezoelectric spark generator. 64

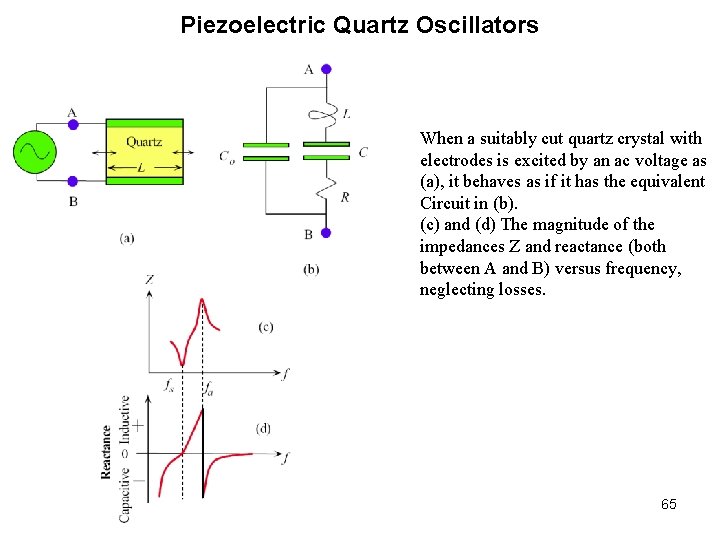
Piezoelectric Quartz Oscillators When a suitably cut quartz crystal with electrodes is excited by an ac voltage as (a), it behaves as if it has the equivalent Circuit in (b). (c) and (d) The magnitude of the impedances Z and reactance (both between A and B) versus frequency, neglecting losses. 65

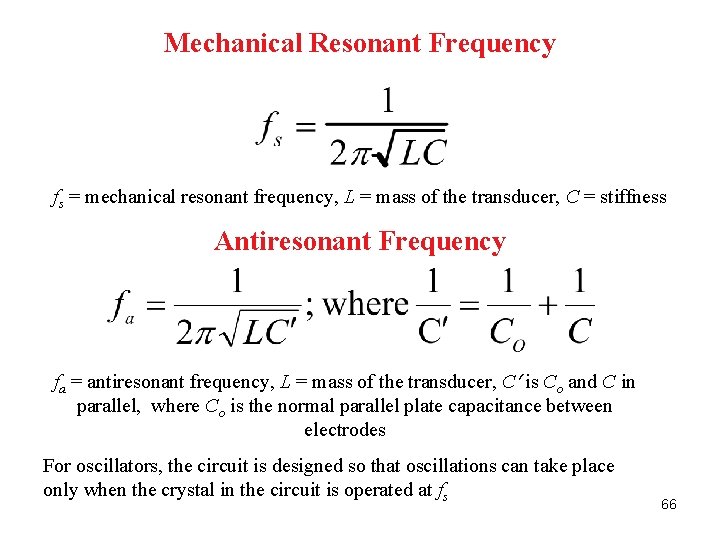
Mechanical Resonant Frequency fs = mechanical resonant frequency, L = mass of the transducer, C = stiffness Antiresonant Frequency fa = antiresonant frequency, L = mass of the transducer, C is Co and C in parallel, where Co is the normal parallel plate capacitance between electrodes For oscillators, the circuit is designed so that oscillations can take place only when the crystal in the circuit is operated at fs 66

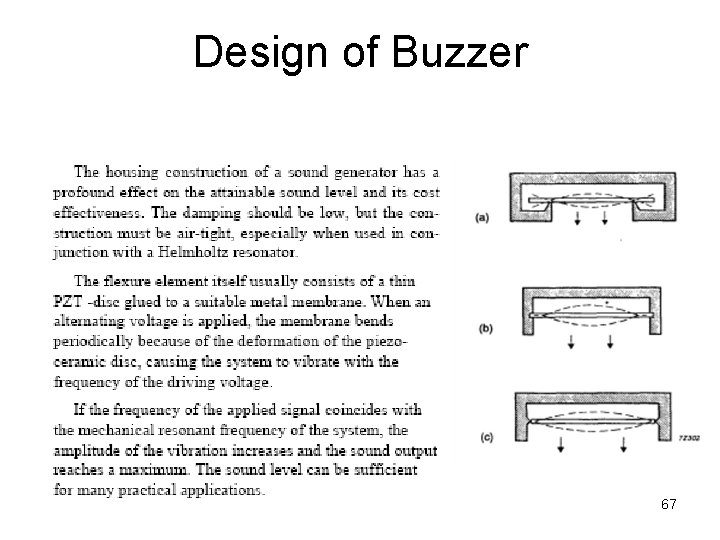
Design of Buzzer 67

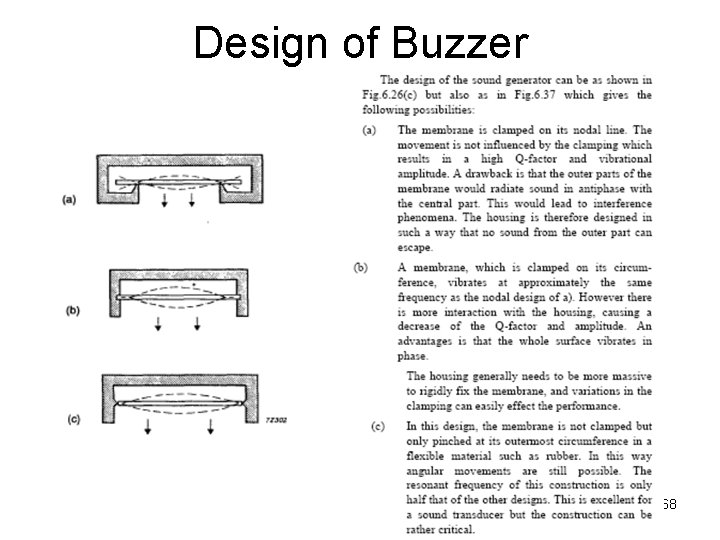
Design of Buzzer 68

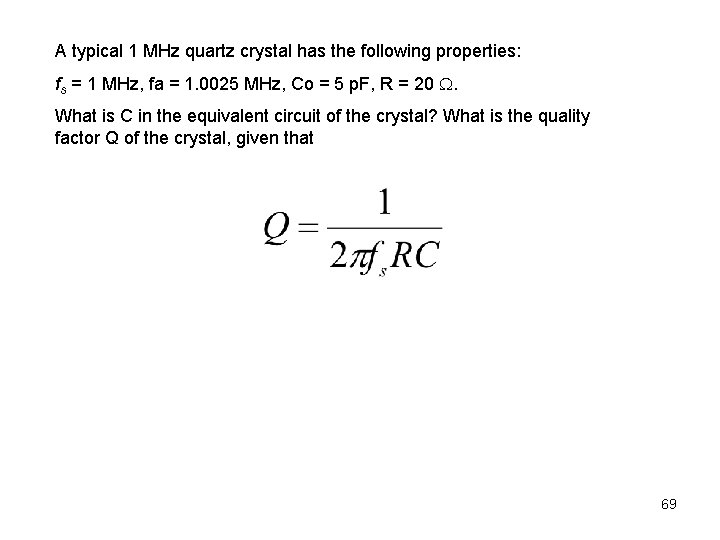
A typical 1 MHz quartz crystal has the following properties: fs = 1 MHz, fa = 1. 0025 MHz, Co = 5 p. F, R = 20 . What is C in the equivalent circuit of the crystal? What is the quality factor Q of the crystal, given that 69

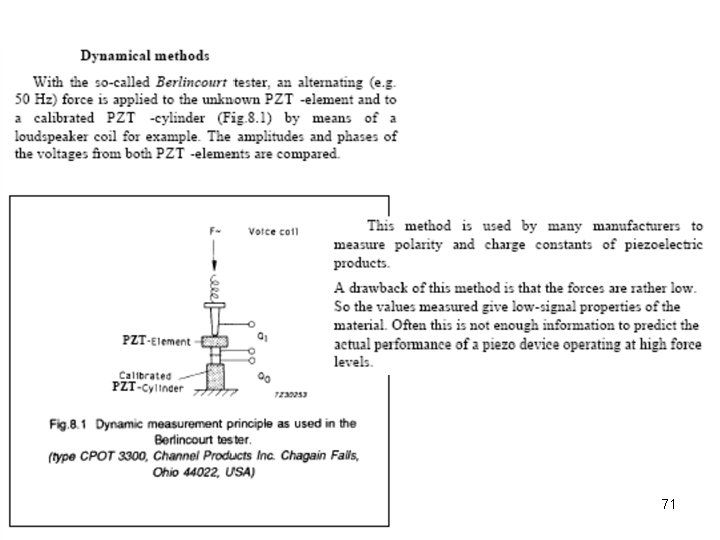
Piezoelectric measurement 70

71
 Heckmann diagram
Heckmann diagram High k dielectric
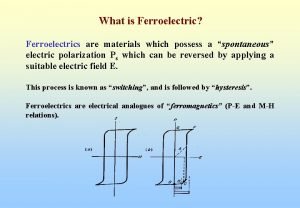
High k dielectric Fram (ferroelectrics ram)
Fram (ferroelectrics ram) Radiant ferroelectric testing
Radiant ferroelectric testing Polarization catastrophe in ferroelectrics
Polarization catastrophe in ferroelectrics Favourite cars
Favourite cars Useful materials at home and their uses
Useful materials at home and their uses Natural man made
Natural man made Adopting materials
Adopting materials Unit 2 energy, materials, systems and devices answers
Unit 2 energy, materials, systems and devices answers Unit 2 energy, materials, systems and devices answers
Unit 2 energy, materials, systems and devices answers Direct materials budget with multiple materials
Direct materials budget with multiple materials Example of literary device
Example of literary device Input and output devices chart
Input and output devices chart Look through the reference material and the map and name
Look through the reference material and the map and name Crown lengthening procedure steps ppt
Crown lengthening procedure steps ppt Introduction to materials science for engineers chapter 10
Introduction to materials science for engineers chapter 10 Product handling definition
Product handling definition Introduction to dental materials
Introduction to dental materials Introduction to materials science for engineers chapter 10
Introduction to materials science for engineers chapter 10 Introduction to materials testing
Introduction to materials testing Introduction to material handling
Introduction to material handling Introduction to poetry billy collins
Introduction to poetry billy collins Introduction to input devices
Introduction to input devices Micr input or output
Micr input or output Comfort devices bed cradle
Comfort devices bed cradle Lesson 5: introduction to electrical devices
Lesson 5: introduction to electrical devices Prom pld
Prom pld Introduction to programmable logic devices
Introduction to programmable logic devices Logic functions
Logic functions Moods in poetry
Moods in poetry Poetic devices repetition
Poetic devices repetition Fixed logic devices
Fixed logic devices Lesson 7 introduction to wiring devices
Lesson 7 introduction to wiring devices Secondary storage devices that use laser technology
Secondary storage devices that use laser technology Lesson 7 introduction to wiring devices
Lesson 7 introduction to wiring devices Purchasing receiving storing and issuing(pdf)
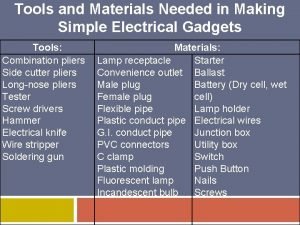
Purchasing receiving storing and issuing(pdf) Simple electrical gadgets examples
Simple electrical gadgets examples Intro paragraph layout
Intro paragraph layout Input vs output devices
Input vs output devices Graphics monitors in computer graphics
Graphics monitors in computer graphics Positive impacts of material technology
Positive impacts of material technology Translucent examples materials
Translucent examples materials Orthotropic material examples
Orthotropic material examples Effects of light on smart and modern materials
Effects of light on smart and modern materials Smart and modern materials
Smart and modern materials Raw material research and development council salary scale
Raw material research and development council salary scale What is the name of the building
What is the name of the building Reggio emilia toys
Reggio emilia toys Setting up trays and trolleys
Setting up trays and trolleys Properties of dental materials
Properties of dental materials Grade 6 natural science matter and materials
Grade 6 natural science matter and materials Matter and materials grade 7
Matter and materials grade 7 Natural science grade 6 term 3
Natural science grade 6 term 3 Stomatex fabric
Stomatex fabric Modern and smart materials
Modern and smart materials Methods media and materials
Methods media and materials Welding definition
Welding definition Materials transported due to erosion
Materials transported due to erosion Principles of wires
Principles of wires Protective packaging and materials handling
Protective packaging and materials handling Chapter 47 laboratory materials and procedures
Chapter 47 laboratory materials and procedures Protective packaging and materials handling
Protective packaging and materials handling Examples of testing tools
Examples of testing tools Building materials fasteners and adhesives
Building materials fasteners and adhesives What is the difference between smart and modern materials
What is the difference between smart and modern materials Fineness modulus
Fineness modulus Man machine money material
Man machine money material Positive impacts of material technology
Positive impacts of material technology Crystalline and non crystalline materials
Crystalline and non crystalline materials Quality control
Quality control Distinguish between magnetic and nonmagnetic materials
Distinguish between magnetic and nonmagnetic materials