Introduction to EU Regulation for Wearables drs nu

Introduction to EU Regulation for Wearables ©drs. nu, April 2016 1

You will learn • Types of wearables • EU directives for wearables • (required # Declarations of Conformity, CE logo’s, • Roles, responsibilities, parties involved • Specific aspects for medical devices ©drs. nu, April 2016 2

Definition According to Wikipedia, the definition of wearable technology states that wearable computers, also known as body-borne computers or wearables are miniature electronic devices that are worn by the bearer under, with or on top of clothing. ©drs. nu, April 2016 3

Intended Use worn by the bearer under, with or on top of clothing; for which purpose? • • • General Purpose (watch/game/agenda) General Wellness (heart rate monitoring) Medical (arrhythmia detection) as defined by claims made (Intended Use) in combination with nature of the device. ©drs. nu, April 2016 4

Intended Use a device is a medical device if it meets the definition medical device’ means any instrument, apparatus, appliance, software, material or other article, whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of: ©drs. nu, April 2016 5

Intended Use — diagnosis, prevention, monitoring, treatment or alleviation of disease, — diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap, — investigation, replacement or modification of the anatomy or of a physiological process, — control of conception, and which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means. ©drs. nu, April 2016 6
It’s a (medical)device It’s a device, typically consisting of • Casing (armbands, textiles to connect to human body) • Electronics (microcomputer, display, bluetooth) • Preloaded firmware • potentially upgraded by App (should be treated as device on it’s own) • Potentially supplemented by Software running on other platform (should be treated as device on it’s own) ©drs. nu, April 2016 7

It’s regulated Legal manufacturer (you !) responsible for compliance • • Have records on file to confirm your organization and your device meets applicable regulations (= directive + guidance + transposition into local law + EN standard). In case of a medical device these records need to be presented to regulatory authorities, either for notification or for approval to allow CE marking. ©drs. nu, April 2016 8

It’s regulated Which records do you need to keep • A so called Declaration of Conformities (Do. C) confirming that device is in accordance with EU directive/regulation mandating a Do. C. • The more directives apply, the more Do. Cs apply • You may need to obtain these Do. Cs from your suppliers. • In case of a medical device detailed design and manufacturing records substantiating safety and performance of intended use, before issuing Do. C. ©drs. nu, April 2016 9

It’s regulated Which records do you need to keep • A so called Declaration of Conformities (Do. C) confirming that device is in accordance with EU directive/regulation mandating a Do. C. • The more directives apply, the more Do. Cs apply • You may need to obtain these Do. Cs from your suppliers. • In case of a medical device detailed design and manufacturing records substantiating safety and performance of intended use, as supplemented by Do. C. ©drs. nu, April 2016 10

It’s regulated Declaration of Conformity Manufacturer: Authorized Representative: Conformity Assessment procedure: Description of device(s) concerned: Name or type and model: Classification: The device(s) are designed and manufactured in the following facilities: I, the undersigned, hereby declare that the device(s) specified above fulfill the relevant Requirements of Directive So-and-So. Place: Date: Sign: ©drs. nu, April 2016 11

It’s regulated Declaration of Conformity Manufacturer: Authorized Representative: Conformity Assessment procedure: Description of device(s) concerned: Name or type and model: Classification: The device(s) are designed and manufactured in the following facilities: I, the undersigned, hereby declare that the device(s) specified above fulfill the relevant Requirements of Directive So-and-So. Place: Date: Sign: who signs when ? ? ©drs. nu, April 2016 12

Which EU regulations? Casing • • • Regulation (EC) No 1907/2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Casing, as well as any other electrical component in device may hold hazardous substances regulated by Directive 2002/95/EC on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Textiles require marking according to Regulation (EU) No 1007/2011 on textile fibre names and related labelling and marking of the fibre composition of textile products. ©drs. nu, April 2016 13

Which EU regulations? Electronics Do. C • It’s electric, so Low Voltage Directive 2006/95/EC relating to electrical equipment designed for use within certain voltage limits applies • Need to be electromagnetic compatible per EMC Directive 2004/108/EC relating to electromagnetic compatibility (in case of medical device, specific requirements per directive 93/42/EEC concerning medical devices apply). ©drs. nu, April 2016 14

Which EU regulations? Electronics • In case electronics communicate wireless Directive Do. C 1999/5 on Radio Equipment and Telecommunications Terminal Equipment applies • To be recycled per Directive 2012/19/EU on waste electrical and electronic equipment (WEEE) <it’s more than adding the wheelie bin symbol; also analyze amount of electronics placed on the marked and proportionally contribute in recycling funding> ©drs. nu, April 2016 15

Which EU regulations? Firmware • Firmware and Software may process personal data which should be dealt with in accordance with Directive 95/46/EC on the protection of individuals with regard to the processing of personal data and on the free movement of such data. Software • Software should be considered as a device on its own. ©drs. nu, April 2016 16

Which EU regulations? If device is a medical device Do. C • MDD directive 93/42/EEC concerning medical devices òr • IVD directive on in vitro diagnostic medical devices òr • AIMD directive 90/385/EEC on active implantable medical devices apply as well (EMC directive and LVD directive don’t apply) ©drs. nu, April 2016 17

It’s regulated over EU directives • Many different directives apply, many Do. Cs may be required, CE logo may represent compliance to more than one directive. • A lot of evidence to be collected (over your suppliers). • Specific processed per country may need to be followed as a result of the transposition of the EU directives into local law. ©drs. nu, April 2016 18

It’s regulated over EU directives • It’s not a one time thing; keep the information current as • • Your device will change Regulations do change (affecting testing to be done, Do. Cs to be revised (in coming months transition periods to drastically revised MDD, RTTE and LVD will start. Authorities may required start of the art updates (at new regulations) as well as periodic updates Competition may be interested…. ©drs. nu, April 2016 19

Many EU directives, even more EN standards • EU directives set generic requirements • Presumption of compliance to these requirements can be achieved by means of complying to EN standards: http: //ec. europa. eu/growth/singlemarket/european-standards/harmonisedstandards/index_en. htm ©drs. nu, April 2016 20

More EU Directives may apply CE = Σ CE Remote controlled robotic arm on wheelchair CE MDD (medical device) CE RTTE (frequency spectrum) Machine Directive (moving parts) WEEE for electronics, Ro. HS for all ©drs. nu, April 2016 21

More EU Directives may apply CE = Σ CE If more then one directive apply, the primairy intended use determines the governing directive. Refer to directives in product labelling ©drs. nu, April 2016 22

EU Medical Device Regulations For medical devices, health care regulatory authorities require either notification or prior approval to allow CE marking for EU market entry safety and performance ©drs. nu, April 2016 23

Medical Device Regulations US safety and effectiveness EU safety and performance safety and effectiveness ©drs. nu, April 2016 24

EU Medical Device Directives Who are the stakeholders? Manufacturer (1) potentially Authorized Representative (1) Notified Body (approx 60) Competent Authority (29) ©drs. nu, April 2016 25

EU Medical Device Directives Responsibilities Stakeholders Notified Body assessement product + associated Quality System (for > Class I) ©drs. nu, April 2016 26

EU Medical Device Directives Responsibilities Stakeholders Competent Authority (CA) • Entity to whom Class I medical devices need to be notified • Safety Inhabitants • Accreditation Notified Body • Decides at conflict Manufacturer / Notified Body • Can withdraw CE mark ©drs. nu, April 2016 27
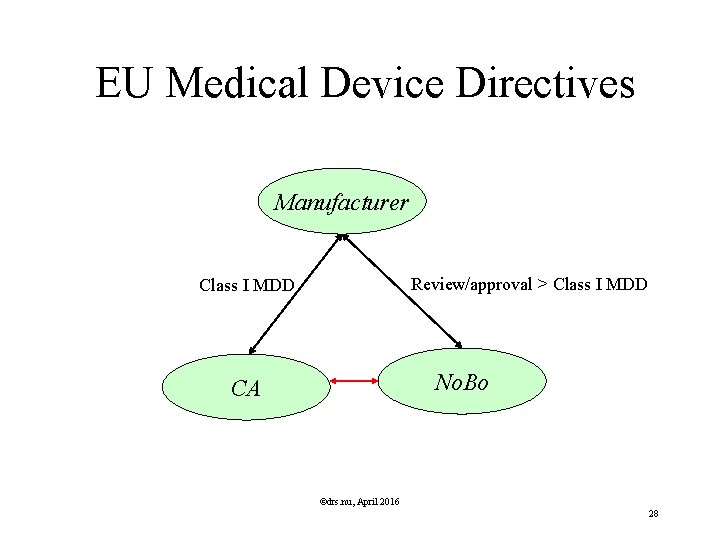
EU Medical Device Directives Manufacturer Review/approval > Class I MDD No. Bo CA ©drs. nu, April 2016 28

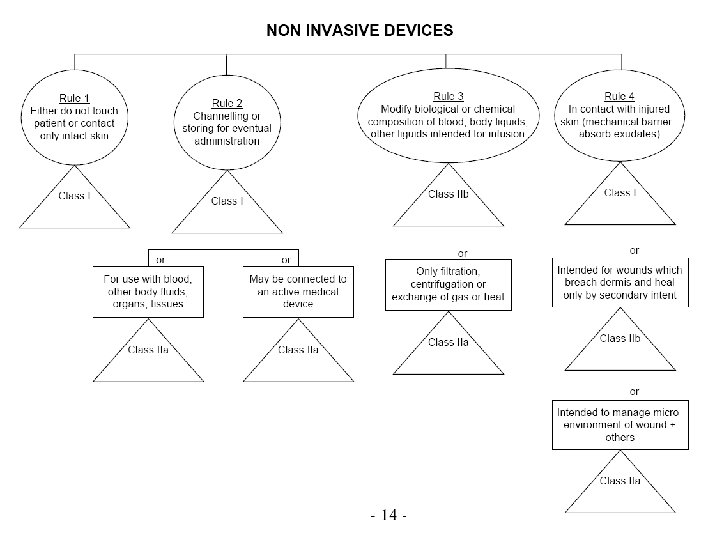
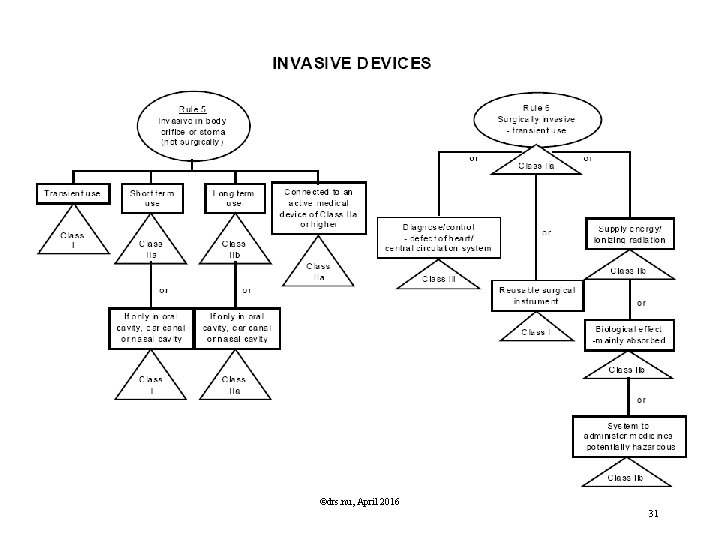
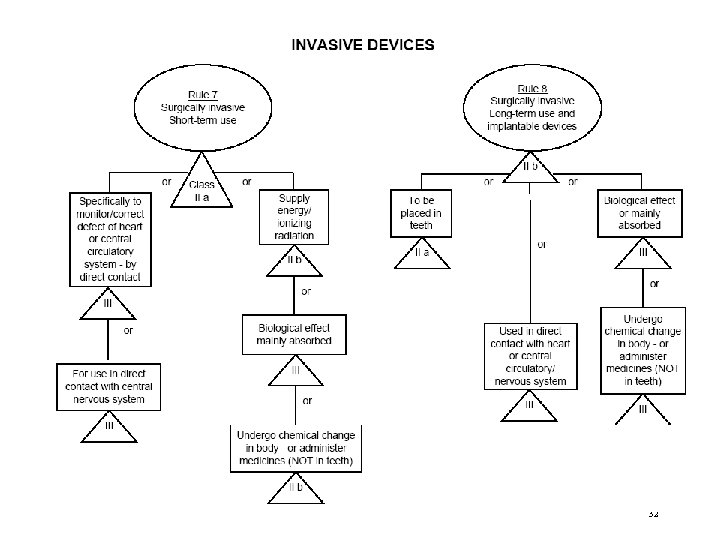
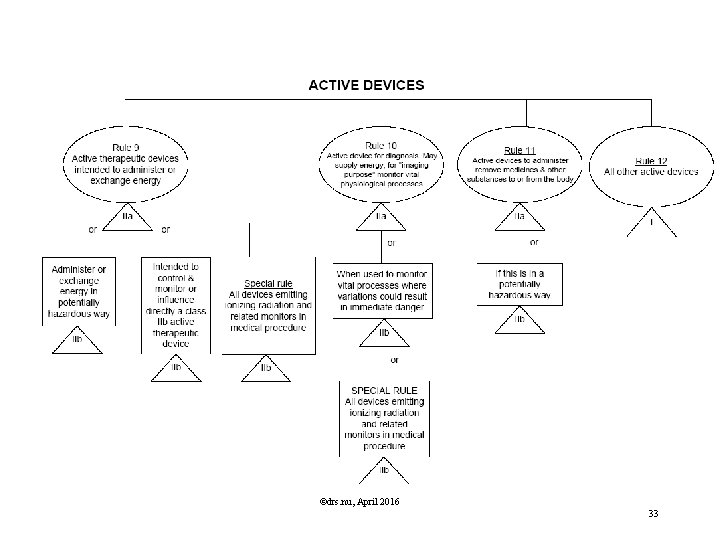
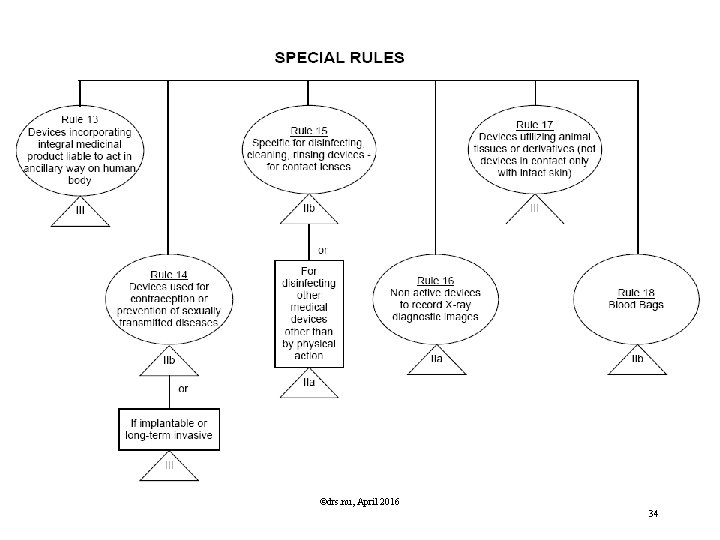
Classification Rules MDD Directive Classification according Annex IX of MDD is based on Duration contact In which manner does the device come into contact? Nature of the Medical Device as defined in the intended use. Resulting in Class I, IIa, IIb or III, Determining the route to CE marking, Determining the regulatory authority involved. ©drs. nu, April 2016 29
©drs. nu, April 2016 30
©drs. nu, April 2016 31
©drs. nu, April 2016 32
©drs. nu, April 2016 33
©drs. nu, April 2016 34
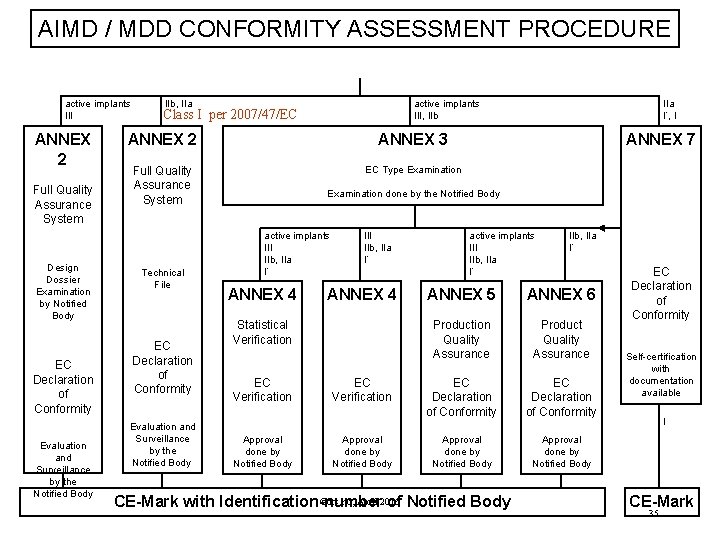
AIMD / MDD CONFORMITY ASSESSMENT PROCEDURE active implants III ANNEX 2 Full Quality Assurance System Design Dossier Examination by Notified Body EC Declaration of Conformity Evaluation and Surveillance by the Notified Body IIb, IIa active implants III, IIb Class I per 2007/47/EC ANNEX 2 ANNEX 3 Full Quality Assurance System EC Type Examination Technical File EC Declaration of Conformity Evaluation and Surveillance by the Notified Body IIa I*, I ANNEX 7 Examination done by the Notified Body active implants III IIb, IIa I* ANNEX 4 Statistical Verification EC Verification Approval done by Notified Body active implants III IIb, IIa I* ANNEX 5 ANNEX 6 Production Quality Assurance Product Quality Assurance EC Declaration of Conformity Approval done by Notified Body ©drs. nu, April 2016 CE-Mark with Identification-number of Notified Body EC Declaration of Conformity Self-certification with documentation available I CE-Mark 35
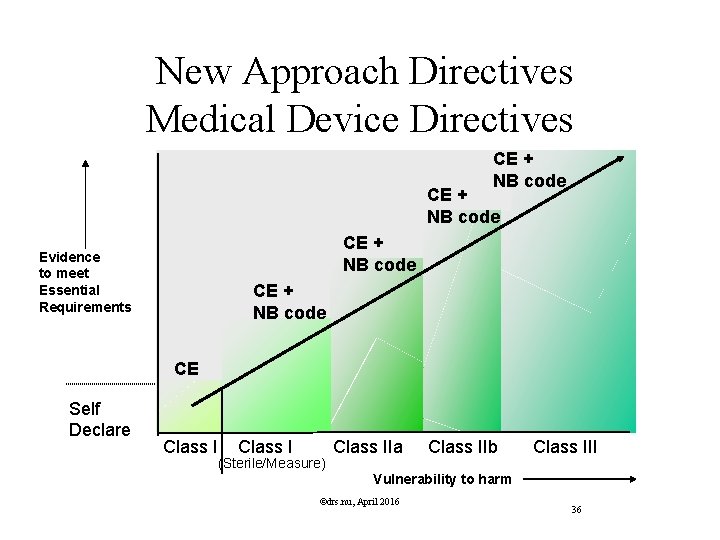
New Approach Directives Medical Device Directives CE + NB code Evidence to meet Essential Requirements CE + NB code CE Self Declare Class I (Sterile/Measure) Class IIa Class IIb Class III Vulnerability to harm ©drs. nu, April 2016 36

Thank you ! ©drs. nu, April 2016 37
- Slides: 37