INTRODUCTION TO EQUILIBRIUM Equilibrium Constant Kc Kp Calculations

INTRODUCTION TO EQUILIBRIUM Equilibrium Constant, Kc & Kp Calculations ICE chart Properties of K Le. Chatelier’s Principle

Equilibrium: ■ a dynamic process in which opposing changes occur at the same rate ■ for reversible processes: the rate of formation of products equals the rate of formation of reactants Homogeneous equilibrium: ■ A process with reactants and products in the same phase ■ For example: H 2(g) + Cl 2(g) ⇌ 2 HCl(g) Heterogeneous equilibrium: ■ A process with reactants and products in different phases: ■ For example: Ca. SO 4(s) ⇌ Ca 2+(aq) + SO 42 -(aq)

Four conditions that apply to all equilibrium systems: 1. Equilibrium is achieved in a reversible process with the rates of opposing changes are equal. 2. The observable (macroscopic) properties of a system at equilibrium are constant. 3. Equilibrium can only be reached in a closed system. For this reason, a system can only be in equilibrium at a constant temperature 4. Equilibrium can be approached from either direction.
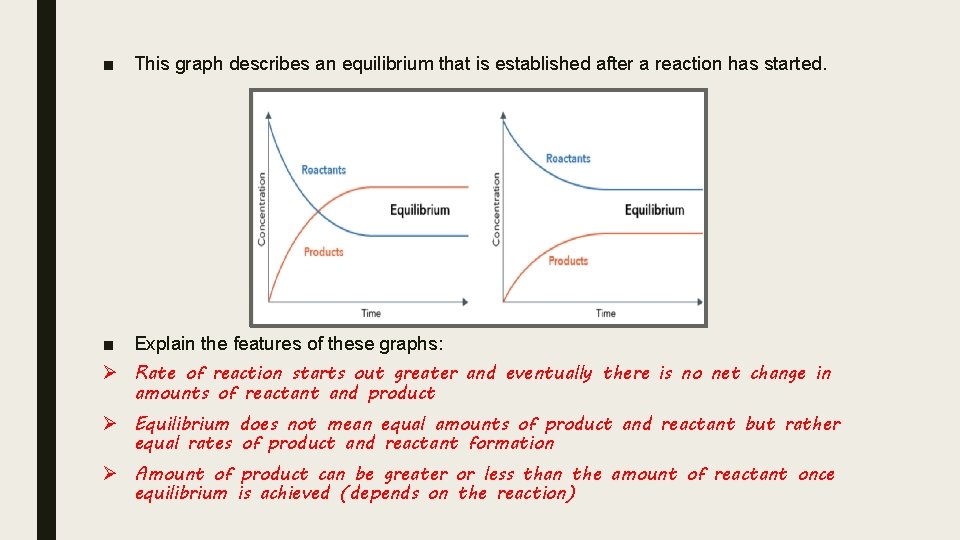
■ This graph describes an equilibrium that is established after a reaction has started. ■ Explain the features of these graphs: Ø Rate of reaction starts out greater and eventually there is no net change in amounts of reactant and product Ø Equilibrium does not mean equal amounts of product and reactant but rather equal rates of product and reactant formation Ø Amount of product can be greater or less than the amount of reactant once equilibrium is achieved (depends on the reaction)
■ AP practice questions

The Equilibrium Constant, Kc ■
The law of chemical equilibrium states that at equilibrium, there is a constant ratio between the concentrations of the products and the reactants. Ø If the value of Kc is very large, what does this tell us about the amount of products as compared to reactants? Ø Very little reactant compared to product once equilibrium is established Ø If the value of Kc is very small, what does this tell us about the amount of products as compared to reactants? Ø Very little product compared to reactant once equilibrium is established Example: CH 3 CO 2 H + H 2 O ⇌ H 3 O+ + CH 3 CO 2 - Ka = 1. 8 x 10 -5 Ø This is acetic acid (vinegar). When in water, an equilibrium is established where very little H 3 O+ is made. That is why vinegar is a weak acid.
■ AP practice questions

Calculations Involving Equilibrium Constant, Kc, and Concentration Example: a) The value of Kc at 698 K for the reaction H 2 + I 2 ⇌ 2 HI, is known to be 1. 84 x-2. 10 If the concentrations of H 2 and I 2 at equilibrium are both 0. 0250 M, what is the concentration of HI?

b) If the initial concentration of H 2 is 0. 100 M and I 2 is 0. 200 M, determine the concentrations of H 2, I 2 and HI at equilibrium.
■ AP practice questions

Calculations Involving Very Small Values of Kc In cases where the value of Kc is very small, this means that very little product is formed. Therefore, the change in concentration of reactant is so small it can, in certain situations, be considered negligible. This will make calculations much easier. Rules for deciding if change in concentration of reactant is negligible: ■ Divide the initial concentration of the reactant by Kc ■ If this value is: > 500 then change in concentration is negligible 100 < 500 then change in concentration may be negligible < 100 then change in concentration is not negligible

Example: At 1100 K, Kc = 4. 20 x 10 -6 for the gas phase reaction, 2 H 2 S(g) ⇌ 2 H 2(g) + S 2(g). What concentration of S 2 can be expected when 0. 200 mole of H 2 S comes to equilibrium at 1100 K in an otherwise empty 1. 00 -L vessel?
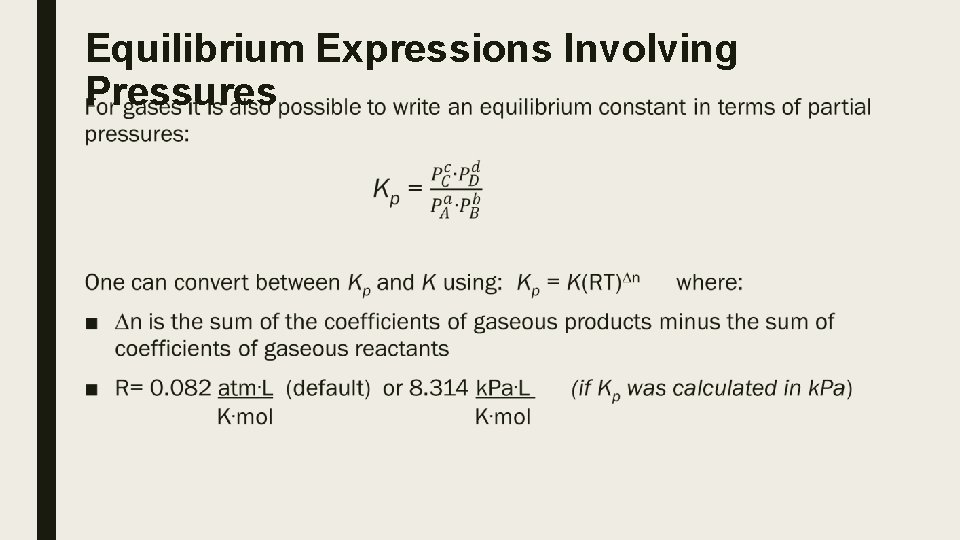
Equilibrium Expressions Involving Pressures ■

Example: For the equilibrium: N 2 O 4(g) ⇌ 2 NO 2(g) (ΔH = +58 k. J mol-1) Kp = 0. 664 at 45 o. C. If the partial pressure of dinitrogen tetroxide was 0. 449 atm, what would be the equilibrium partial pressure of nitrogen dioxide and the total pressure of the gases? What is Kc?
■ AP practice questions
Heterogeneous Equilibria When writing equilibrium expressions, special care must be taken if it is a heterogeneous equilibrium. Experiments have shown that the position of a heterogeneous equilibrium does not depend on the amounts of pure solids or liquids present. As such, they are not included in the Kc expression. For example: Ca. SO 4(s) ⇌ Ca 2+(aq) + SO 42 -(aq) [Ca 2+(aq)][SO 42 -(aq)] Example: Write the expression for Kc and Kp for the equilibrium: Ca. CO 3(s) ⇌ Ca. O (s) + CO 2(g) Kc =
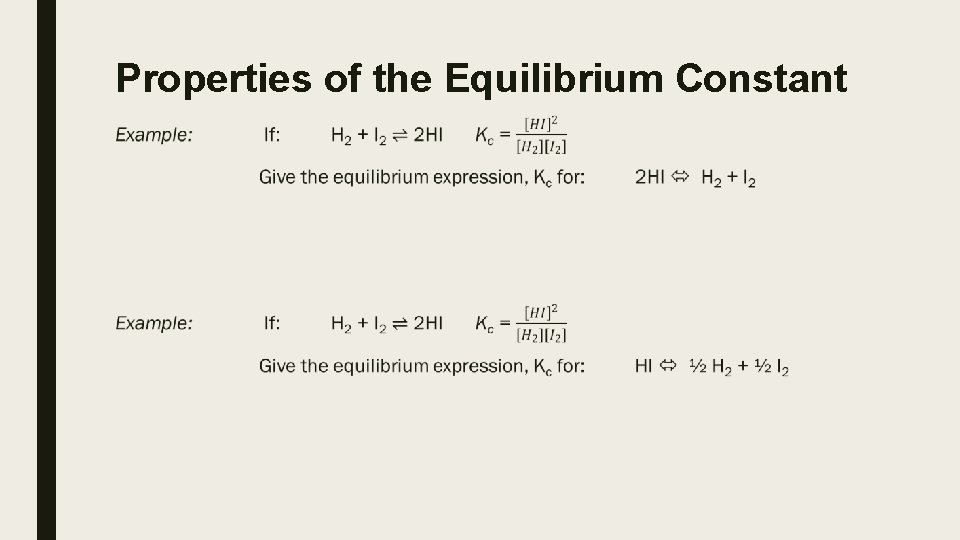
Properties of the Equilibrium Constant ■

Therefore, we can see that: 1. If a reaction at equilibrium is reversed, then the equilibrium constant for that reaction is the reciprocal of the equilibrium constant of the forward reaction. 2. If a reaction at equilibrium has its stoichiometric coefficients altered by a factor c, then the equilibrium constant for that reaction is the equilibrium constant of the original reaction raised to the power of c. 3. When adding reactions at equilibrium, K of the overall resulting reaction is product of the Ks for the reactions that were summed.
■ AP practice questions
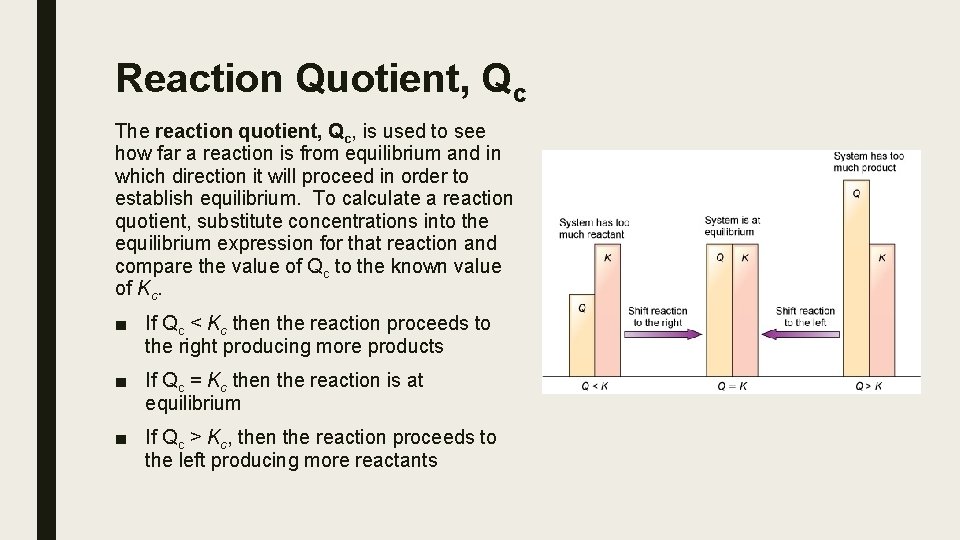
Reaction Quotient, Qc The reaction quotient, Qc, is used to see how far a reaction is from equilibrium and in which direction it will proceed in order to establish equilibrium. To calculate a reaction quotient, substitute concentrations into the equilibrium expression for that reaction and compare the value of Qc to the known value of Kc. ■ If Qc < Kc then the reaction proceeds to the right producing more products ■ If Qc = Kc then the reaction is at equilibrium ■ If Qc > Kc, then the reaction proceeds to the left producing more reactants

Example: H 2 + I 2 ⇌ 2 HI has. Kc = 1. 84 x 10 -2. A flask contains 1. 00 M of H 2, I 2 and HI each. Is this at equilibrium? If not, which reaction (to the left or to the right) will be favored in order to achieve equilibrium?
■ AP practice questions

Le Chatelier’s Principle “If the equilibrium of a system is upset, the system will tend to react in a direction that opposes the change and reestablishes equilibrium. ”

Effect of adding or removing a reactant or product: Example: N 2(g) + 3 H 2(g) ⇌ 2 NH 3(g) assume that this reaction is at equilibrium ■ What would happen if more N 2 was added to the system? Ø Shift right; more product formed, reactants used up ■ What would happen if H 2 was removed from the system? Ø Shift left; more reactant formed, products used up
Change of Temperature: adding or removing Ifheat: you think of the enthalpy as a product or reactant (write it in the equation) it is easy to see how changing the temperature will affect the direction of the reaction. Example: N 2(g) + O 2(g) ⇌ 2 NO(g) DH = 180. 5 k. J What effect would increasing the temperature have? Ø Temperature increase would favor the endothermic reaction, ie the forward reaction, so more product would form and reactant would be used up In general: Ø Endothermic (DH>0): increase of temperature shifts rxn to the right; value of Kc increases Ø Exothermic (DH<0): increase of temperature shifts rxn to the left; value of Kc decreases Ø And vice versa

Effects of Volume and Pressure (Gases): When the volume of a gas is decreased, this has the same effect as increasing the pressure. Gaseous equilibria will adjust in order to relieve this change by reducing the number of molecules present. If the volume is increased (the pressure decreased) the opposite will happen. The value of Kc will not change (as long as there is no change in T). Example: N 2(g) + 3 H 2(g) ⇌ 2 NH 3(g) What effect would decreasing the pressure have on this equilibrium? Ø Decreasing pressure will favor the side with more moles of gas, ie the reactant side. Therefore, sift left, more reactants form and less products are present

Effect of a Catalyst: Since a catalyst works by lowering Ea for both the forward and reverse reaction, the addition of a catalyst does not affect the position of the equilibrium. However, since the catalyst speeds up the rate the reactions occur, the equilibrium will be established faster.

Le Chatelier Activity
■ AP practice questions
- Slides: 30