INTRODUCTION TO DNA DR DEA THOMAS MOLECULAR MEDICINE













- Slides: 62

INTRODUCTION TO DNA DR DEA THOMAS

MOLECULAR MEDICINE STUDY OF THE PHYSIOLOGY AND PATHOLOGY OF THE GENETIC MATERIAL IN A CELL

OBJECTIVES Genetic information Central Dogma Packaging DNA Structure Nucleotide Synthesis Purine Metabolism Clinical Correlations Pyrimidine Metabolism Clinical Correlations Pharmacological Applications

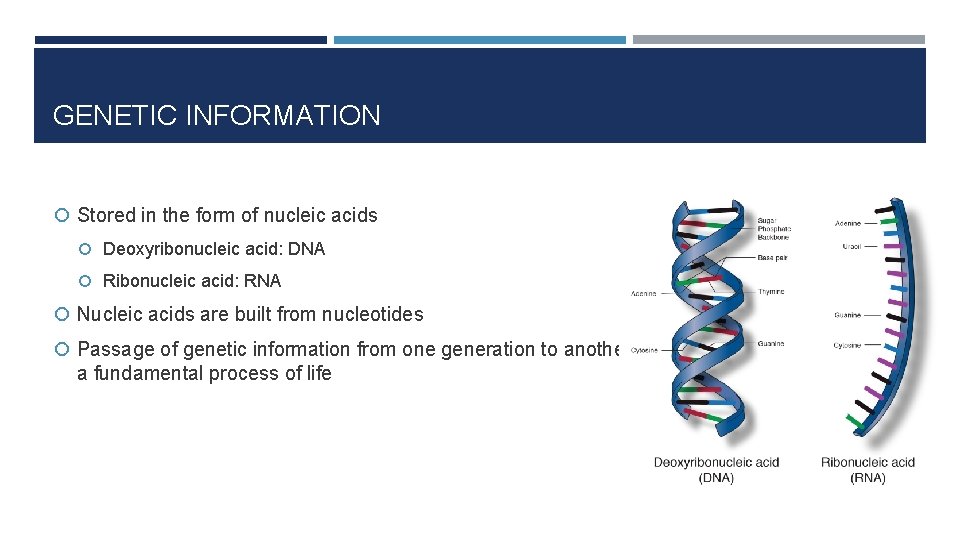
GENETIC INFORMATION Stored in the form of nucleic acids Deoxyribonucleic acid: DNA Ribonucleic acid: RNA Nucleic acids are built from nucleotides Passage of genetic information from one generation to another is a fundamental process of life

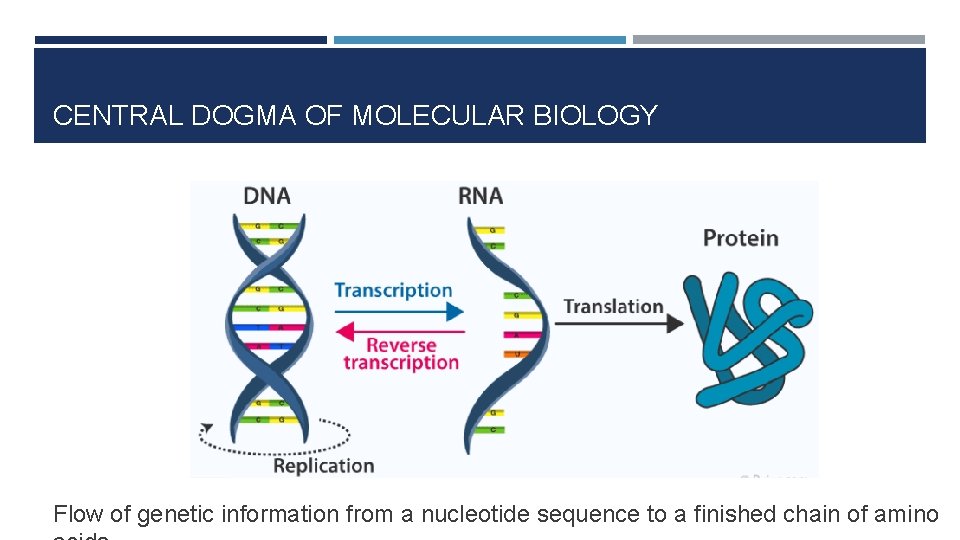
CENTRAL DOGMA OF MOLECULAR BIOLOGY Flow of genetic information from a nucleotide sequence to a finished chain of amino

GENETIC INFORMATION Gene A DNA segment that is the basic unit of genetic information Codes for functional RNA products – not all become final proteins Genes are packaged into chromosomes found in the nucleus of a cell Complete complement of genes: genome

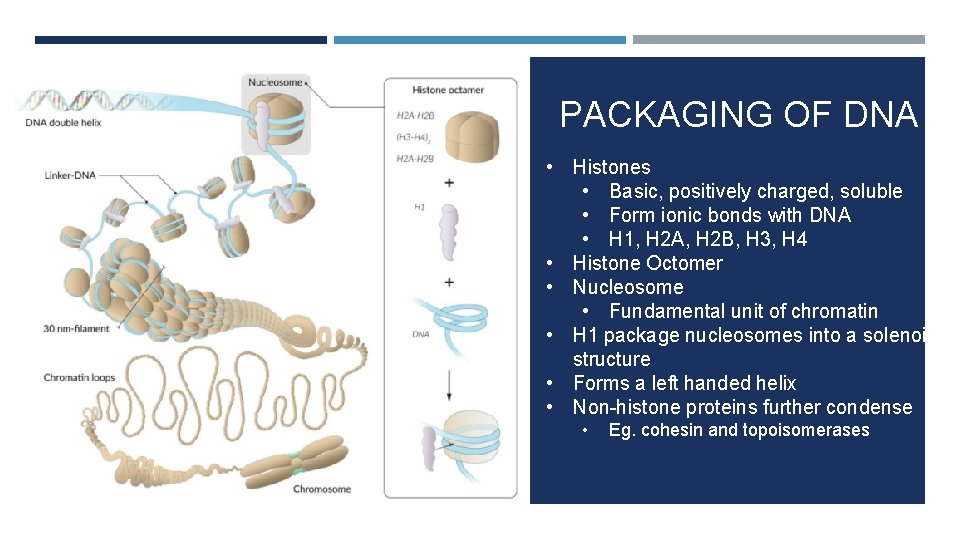
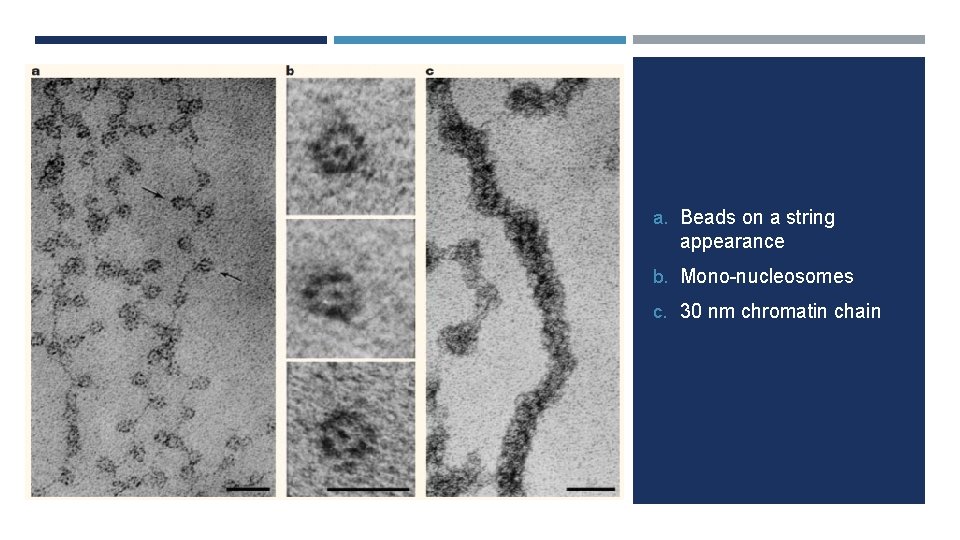
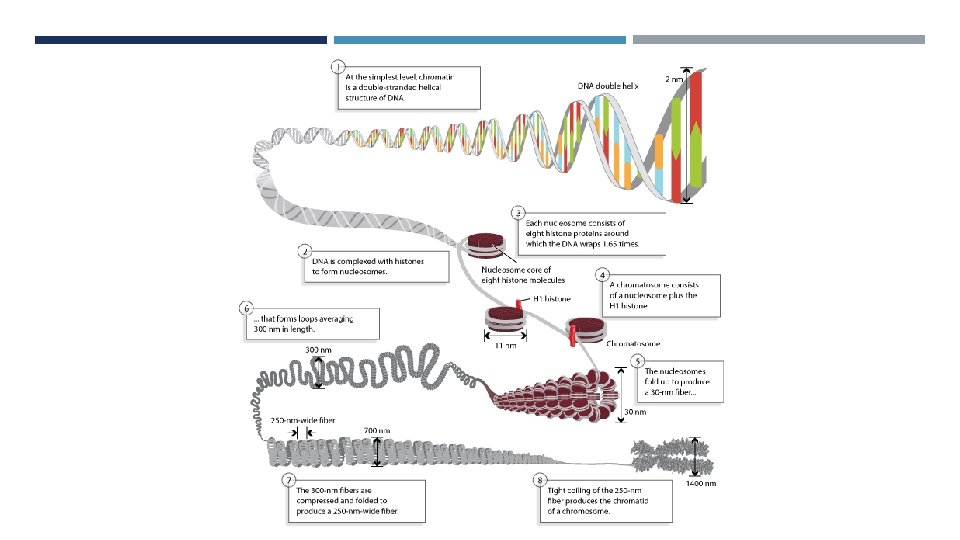
PACKAGING OF DNA • Histones • Basic, positively charged, soluble • Form ionic bonds with DNA • H 1, H 2 A, H 2 B, H 3, H 4 • Histone Octomer • Nucleosome • Fundamental unit of chromatin • H 1 package nucleosomes into a solenoid structure • Forms a left handed helix • Non-histone proteins further condense • Eg. cohesin and topoisomerases

a. Beads on a string appearance b. Mono-nucleosomes c. 30 nm chromatin chain

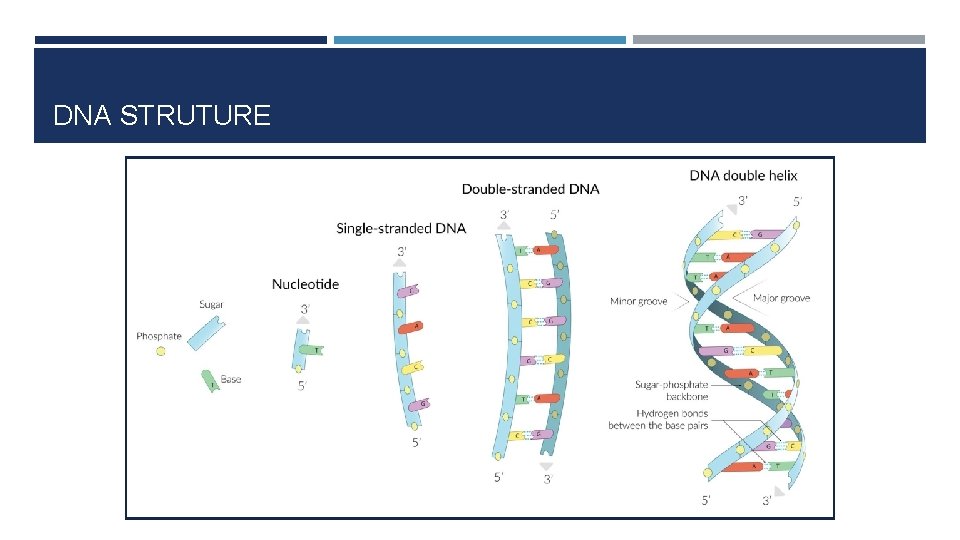
DNA STRUTURE

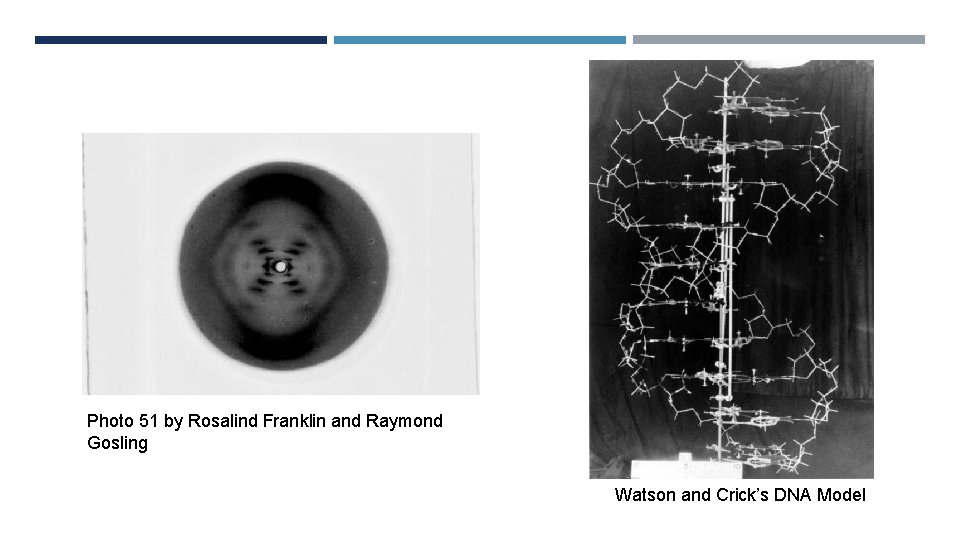
Photo 51 by Rosalind Franklin and Raymond Gosling Watson and Crick’s DNA Model

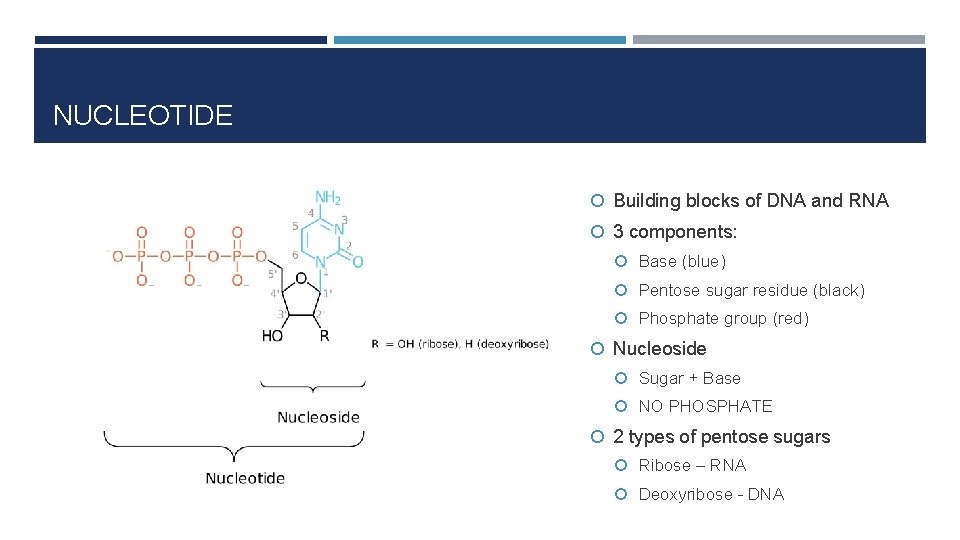
NUCLEOTIDE Building blocks of DNA and RNA 3 components: Base (blue) Pentose sugar residue (black) Phosphate group (red) Nucleoside Sugar + Base NO PHOSPHATE 2 types of pentose sugars Ribose – RNA Deoxyribose - DNA

FUNCTIONS OF NUCLEOTIDES Energy currency in metabolism. Eg. ATP 2 nd messenger systems eg. c. AMP Metabolic intermediates eg. UDP-glucose in glucuneogensis BUILDING BLOCKS OF NUCLEIC ACIDS

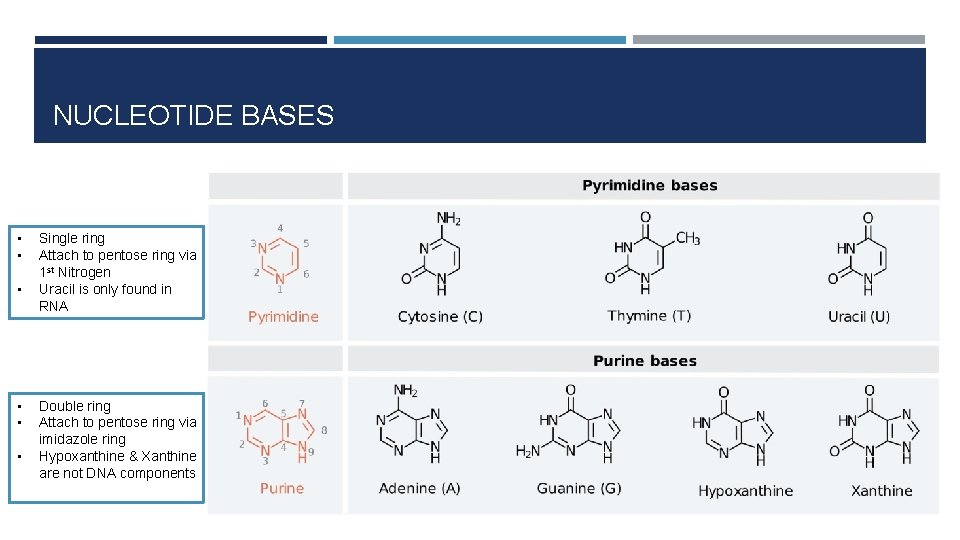
NUCLEOTIDE BASES • • • Single ring Attach to pentose ring via 1 st Nitrogen Uracil is only found in RNA Double ring Attach to pentose ring via imidazole ring Hypoxanthine & Xanthine are not DNA components

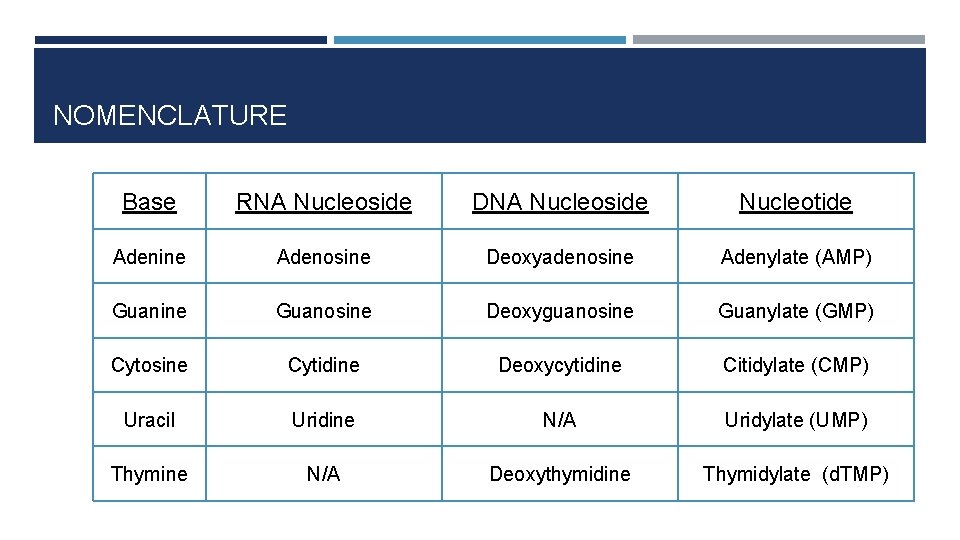
NOMENCLATURE Base RNA Nucleoside DNA Nucleoside Nucleotide Adenine Adenosine Deoxyadenosine Adenylate (AMP) Guanine Guanosine Deoxyguanosine Guanylate (GMP) Cytosine Cytidine Deoxycytidine Citidylate (CMP) Uracil Uridine N/A Uridylate (UMP) Thymine N/A Deoxythymidine Thymidylate (d. TMP)

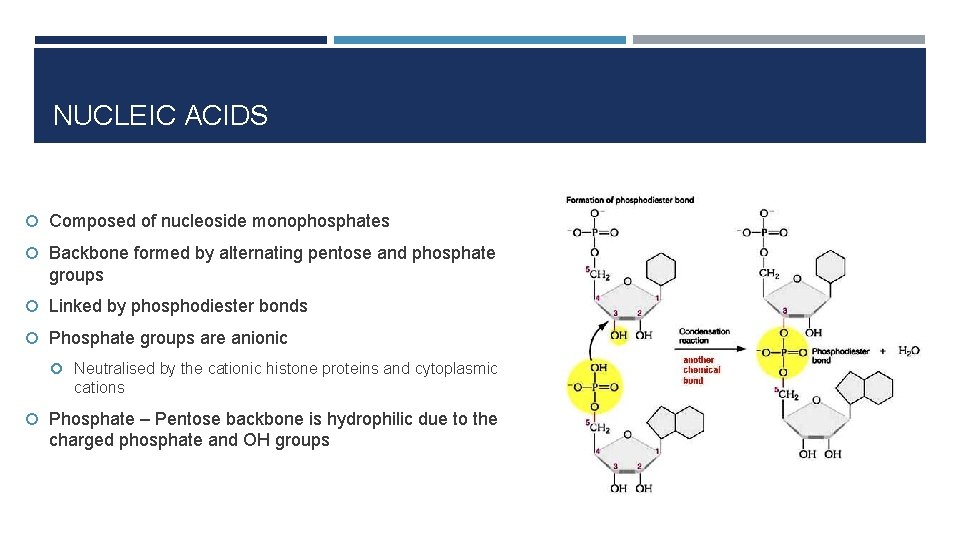
NUCLEIC ACIDS Composed of nucleoside monophosphates Backbone formed by alternating pentose and phosphate groups Linked by phosphodiester bonds Phosphate groups are anionic Neutralised by the cationic histone proteins and cytoplasmic cations Phosphate – Pentose backbone is hydrophilic due to the charged phosphate and OH groups

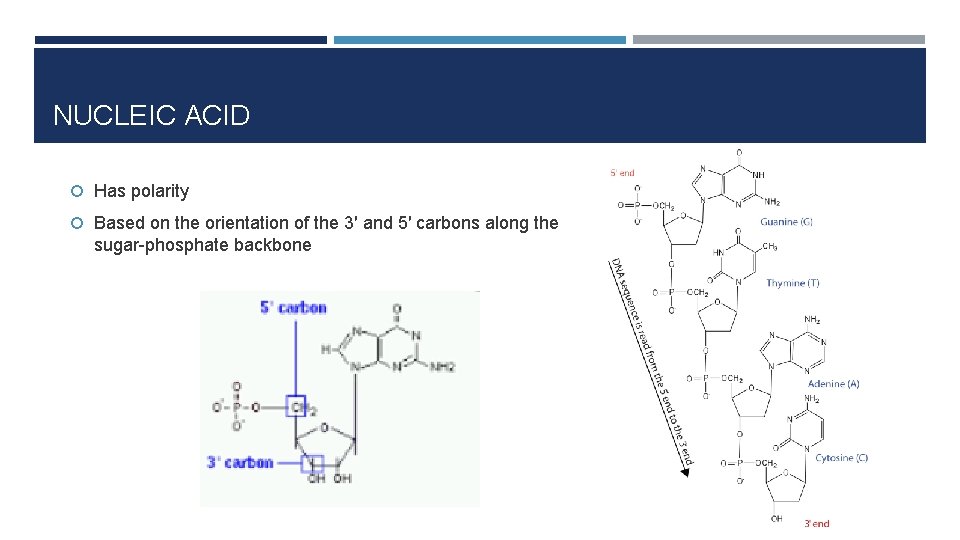
NUCLEIC ACID Has polarity Based on the orientation of the 3′ and 5′ carbons along the sugar-phosphate backbone

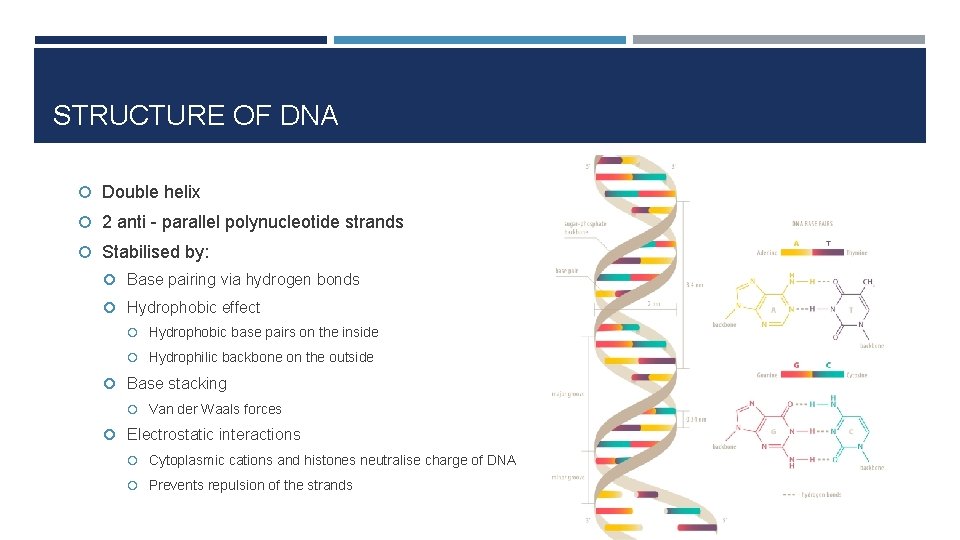
STRUCTURE OF DNA Double helix 2 anti - parallel polynucleotide strands Stabilised by: Base pairing via hydrogen bonds Hydrophobic effect Hydrophobic base pairs on the inside Hydrophilic backbone on the outside Base stacking Van der Waals forces Electrostatic interactions Cytoplasmic cations and histones neutralise charge of DNA Prevents repulsion of the strands

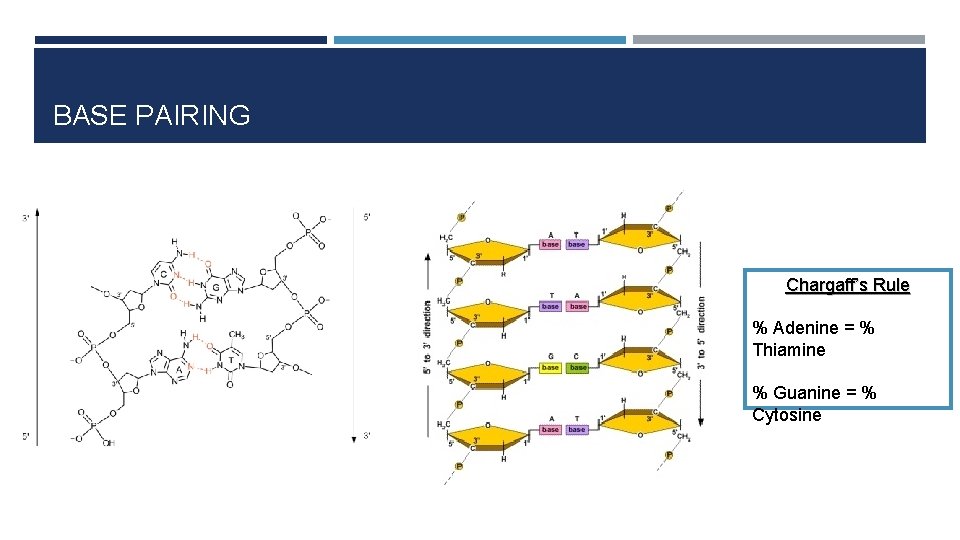
BASE PAIRING Chargaff’s Rule % Adenine = % Thiamine % Guanine = % Cytosine

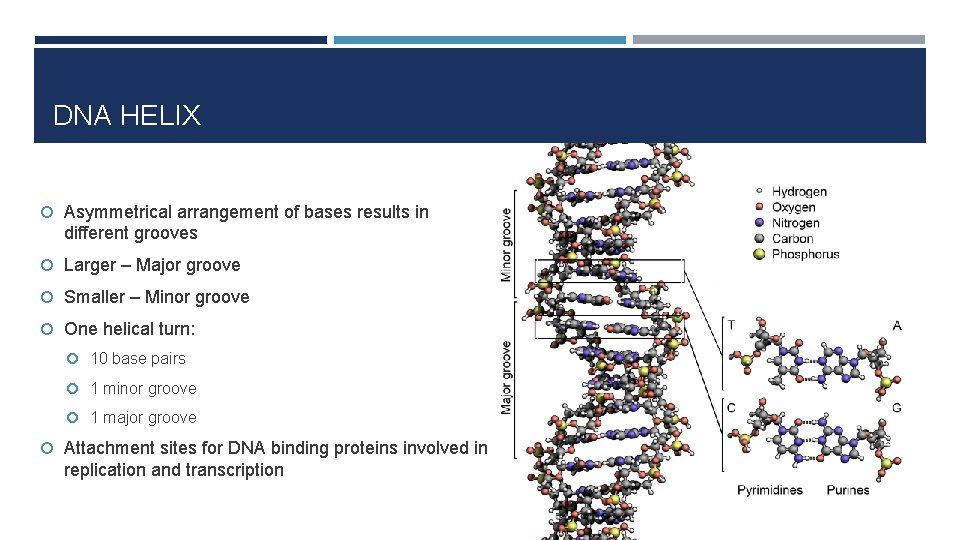
DNA HELIX Asymmetrical arrangement of bases results in different grooves Larger – Major groove Smaller – Minor groove One helical turn: 10 base pairs 1 minor groove 1 major groove Attachment sites for DNA binding proteins involved in replication and transcription


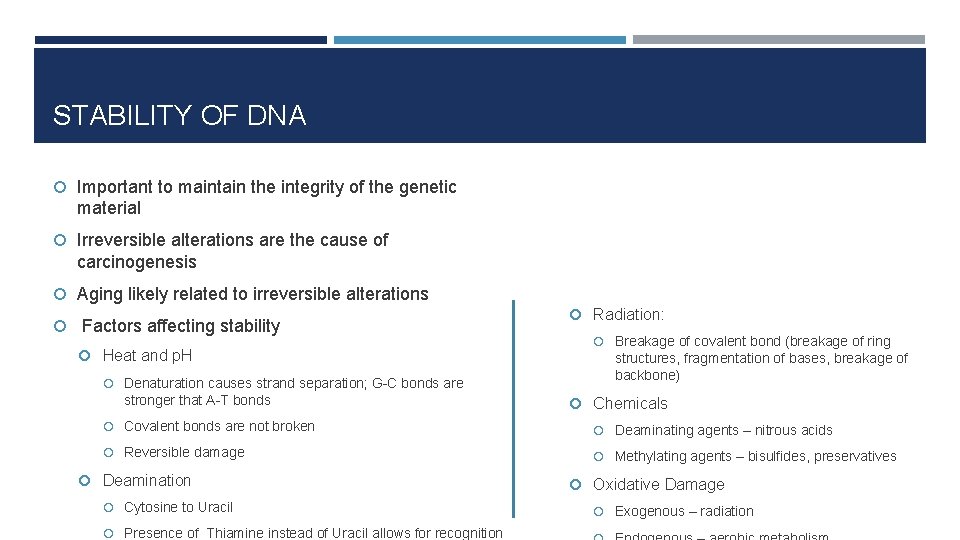
STABILITY OF DNA Important to maintain the integrity of the genetic material Irreversible alterations are the cause of carcinogenesis Aging likely related to irreversible alterations Factors affecting stability Heat and p. H Denaturation causes strand separation; G-C bonds are stronger that A-T bonds Radiation: Breakage of covalent bond (breakage of ring structures, fragmentation of bases, breakage of backbone) Chemicals Covalent bonds are not broken Deaminating agents – nitrous acids Reversible damage Methylating agents – bisulfides, preservatives Deamination Cytosine to Uracil Presence of Thiamine instead of Uracil allows for recognition Oxidative Damage Exogenous – radiation

NUCLEOTIDE SYNTHESIS

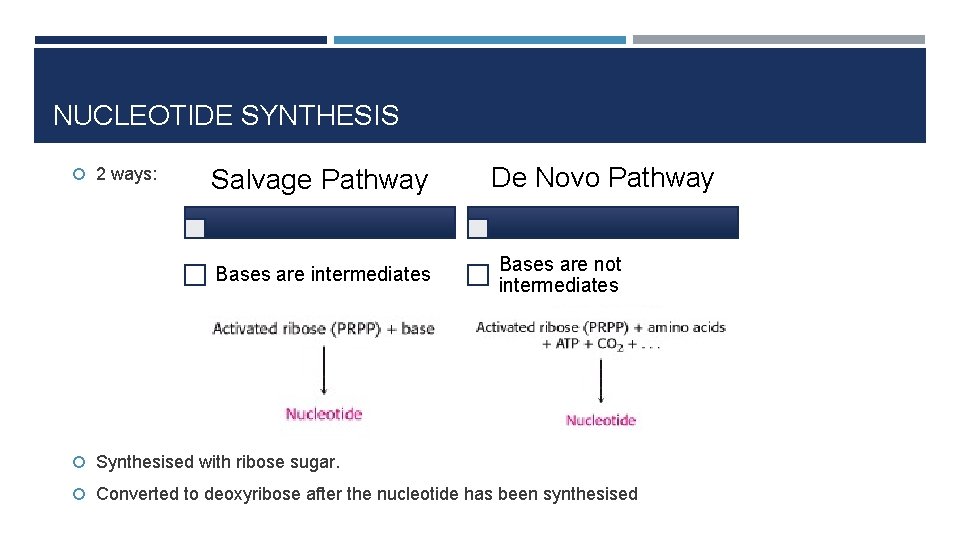
NUCLEOTIDE SYNTHESIS 2 ways: Salvage Pathway Bases are intermediates De Novo Pathway Bases are not intermediates Synthesised with ribose sugar. Converted to deoxyribose after the nucleotide has been synthesised

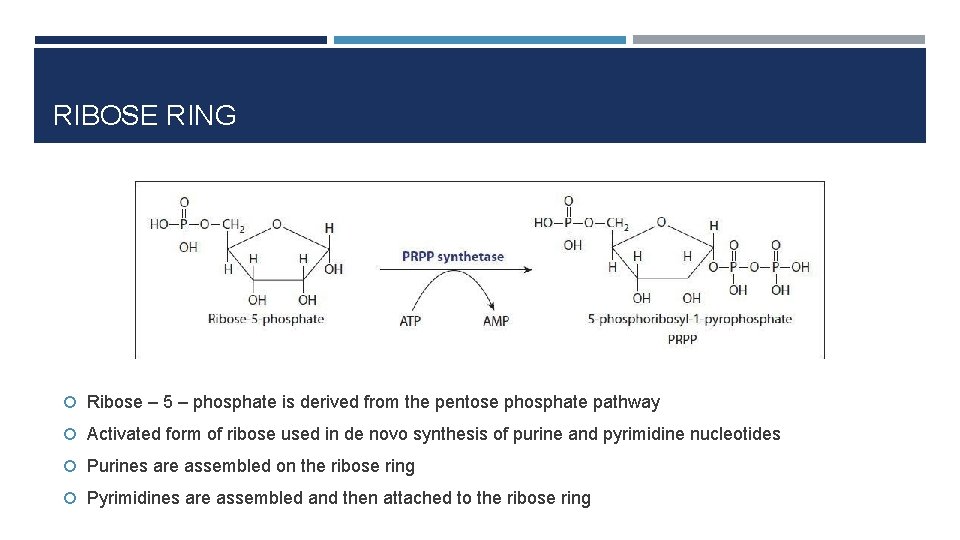
RIBOSE RING Ribose – 5 – phosphate is derived from the pentose phosphate pathway Activated form of ribose used in de novo synthesis of purine and pyrimidine nucleotides Purines are assembled on the ribose ring Pyrimidines are assembled and then attached to the ribose ring

PURINE METABOLISM

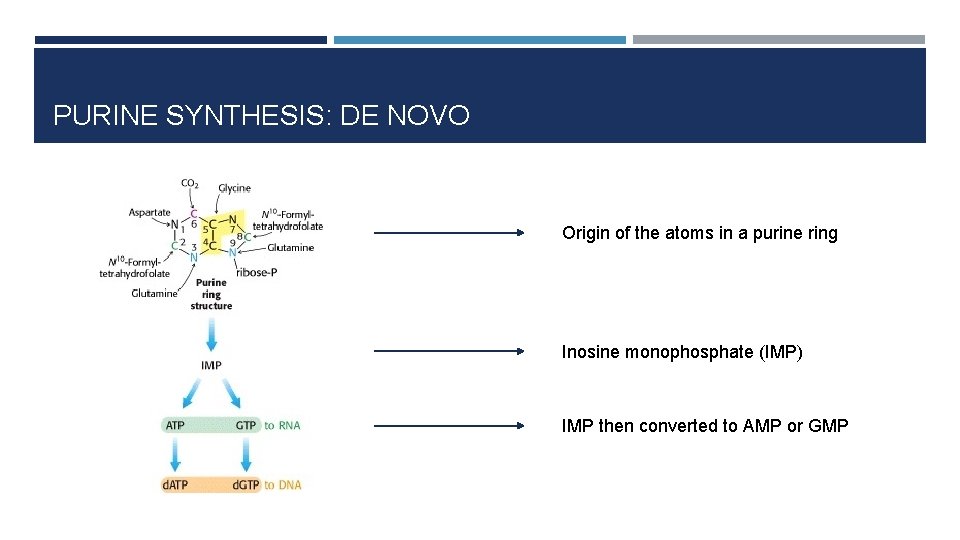
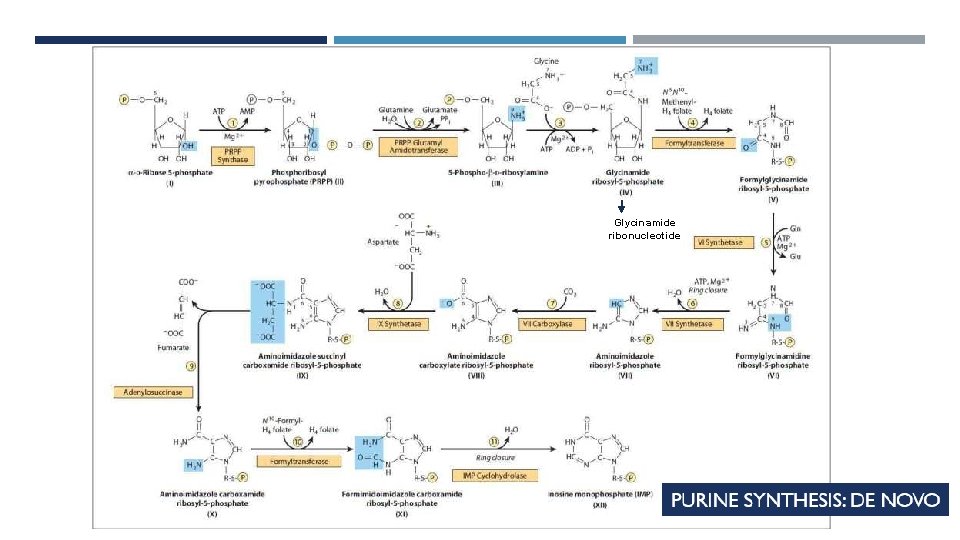
PURINE SYNTHESIS: DE NOVO Origin of the atoms in a purine ring Inosine monophosphate (IMP) IMP then converted to AMP or GMP

PURINE SYNTHESIS Glycinamide ribonucleotide


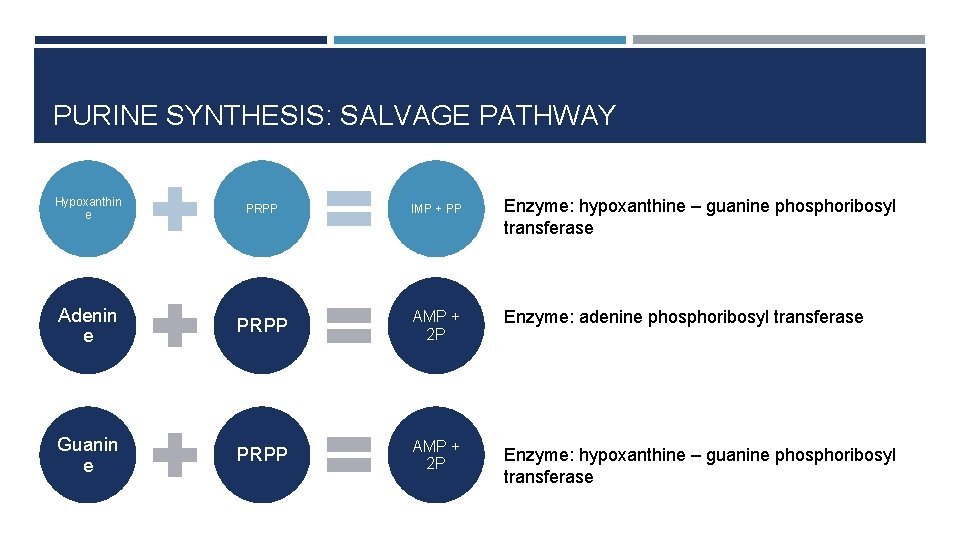
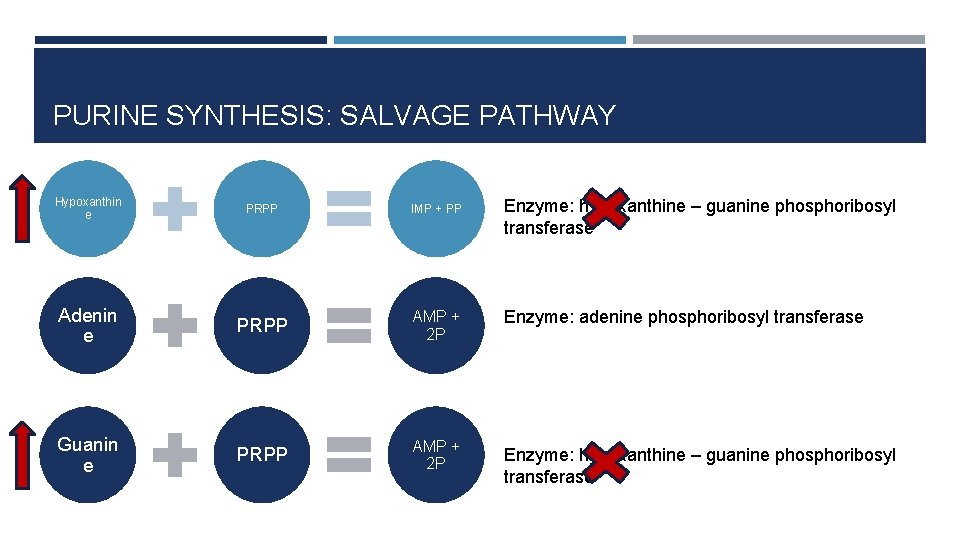
PURINE SYNTHESIS: SALVAGE PATHWAY Hypoxanthin e PRPP IMP + PP Adenin e PRPP AMP + 2 P Guanin e PRPP AMP + 2 P Enzyme: hypoxanthine – guanine phosphoribosyl transferase Enzyme: adenine phosphoribosyl transferase Enzyme: hypoxanthine – guanine phosphoribosyl transferase

REGULATION OF PURINE SYNTHESIS Regulation is needed for 2 reasons: Prevent wastage of energy Prevent build of metabolic waste products 4 main mechanisms

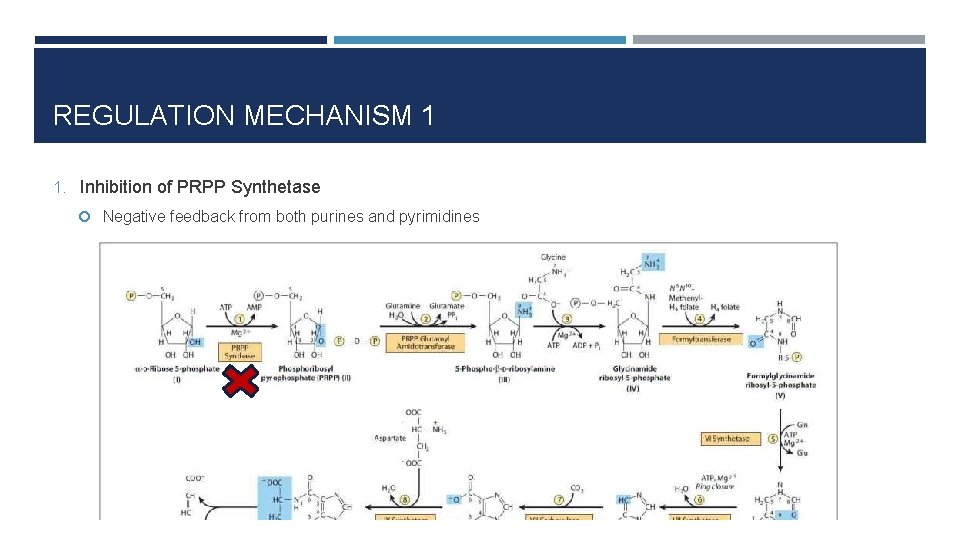
REGULATION MECHANISM 1 1. Inhibition of PRPP Synthetase Negative feedback from both purines and pyrimidines

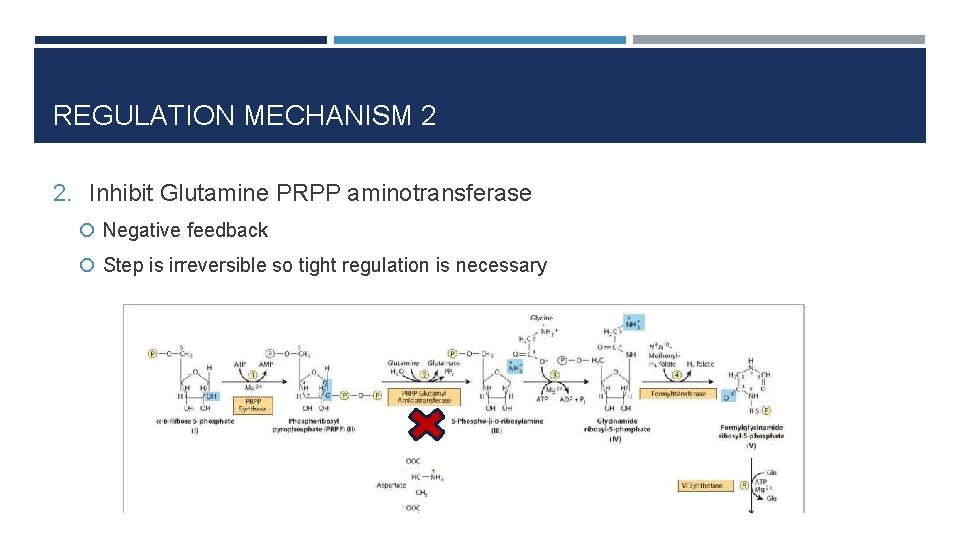
REGULATION MECHANISM 2 2. Inhibit Glutamine PRPP aminotransferase Negative feedback Step is irreversible so tight regulation is necessary

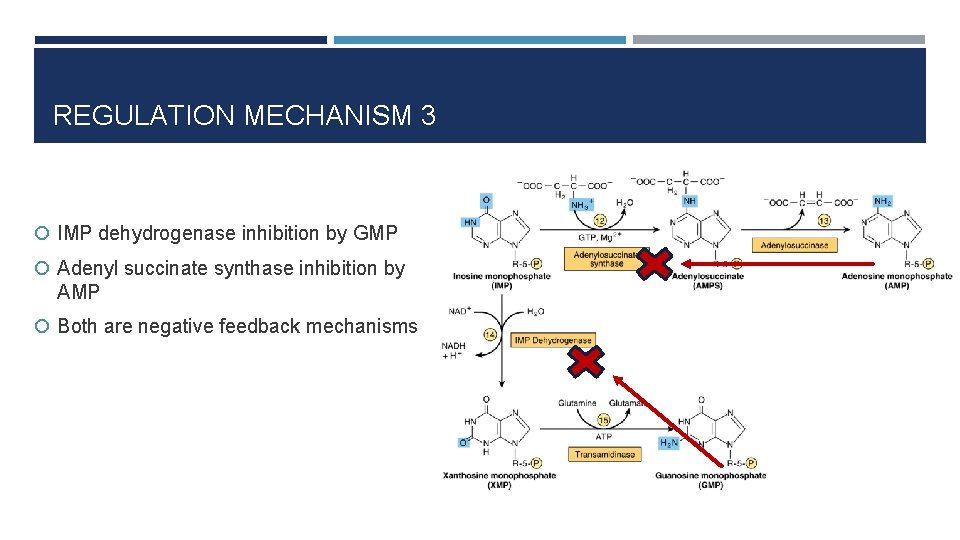
REGULATION MECHANISM 3 IMP dehydrogenase inhibition by GMP Adenyl succinate synthase inhibition by AMP Both are negative feedback mechanisms

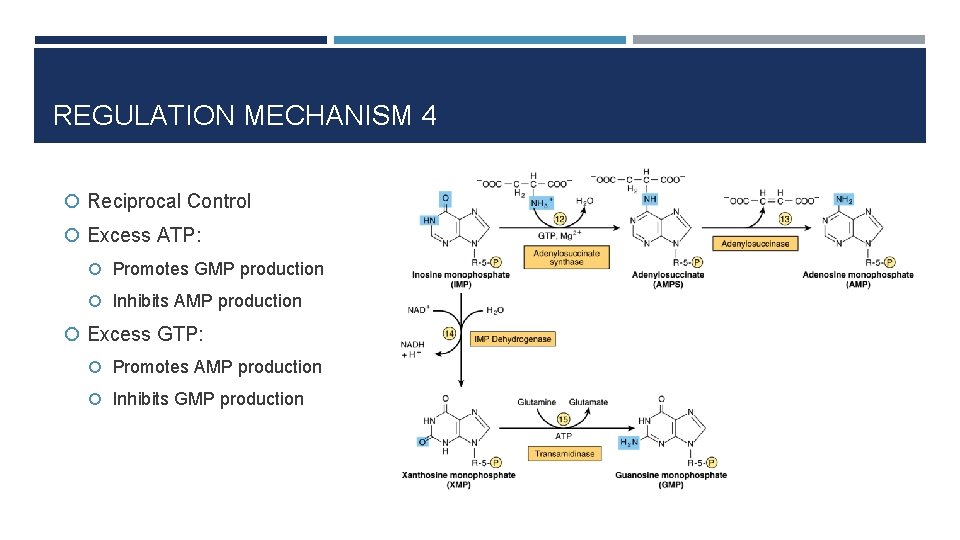
REGULATION MECHANISM 4 Reciprocal Control Excess ATP: Promotes GMP production Inhibits AMP production Excess GTP: Promotes AMP production Inhibits GMP production

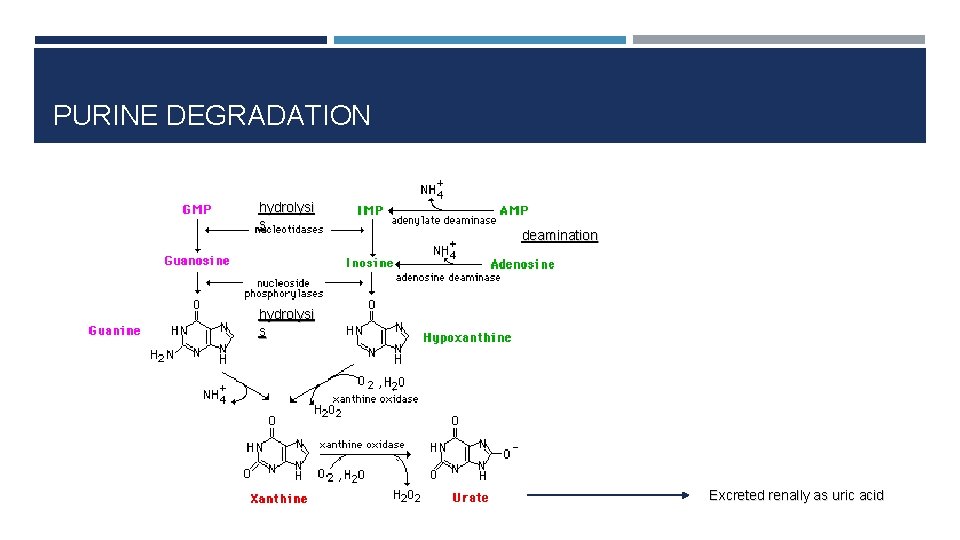
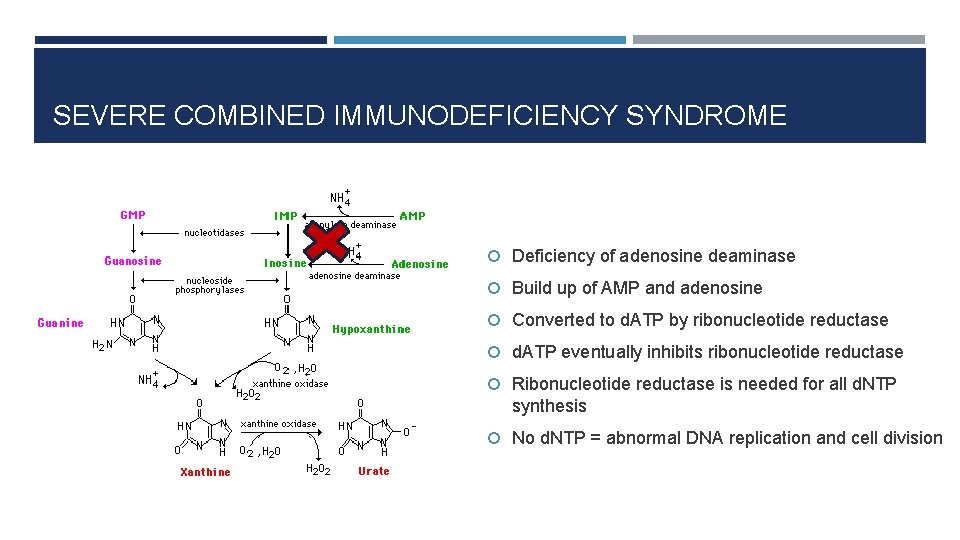
PURINE DEGRADATION hydrolysi s deamination hydrolysi s Excreted renally as uric acid

CLINICAL CORRELATION Defects in purine metabolism can lead to several disorders Severe combined immune deficiency Hyperuricemia and gout Hypermetabolic states Alcoholism G 6 PD Deficiency Lesch-Nyam Syndrome Partial HGRT Deficiency Hereditary – fructose intolerance Galactose – 1 – phosphate uridyl transferase deficiency (galactosemia)

SEVERE COMBINED IMMUNODEFICIENCY SYNDROME Deficiency of adenosine deaminase Build up of AMP and adenosine Converted to d. ATP by ribonucleotide reductase d. ATP eventually inhibits ribonucleotide reductase Ribonucleotide reductase is needed for all d. NTP synthesis No d. NTP = abnormal DNA replication and cell division

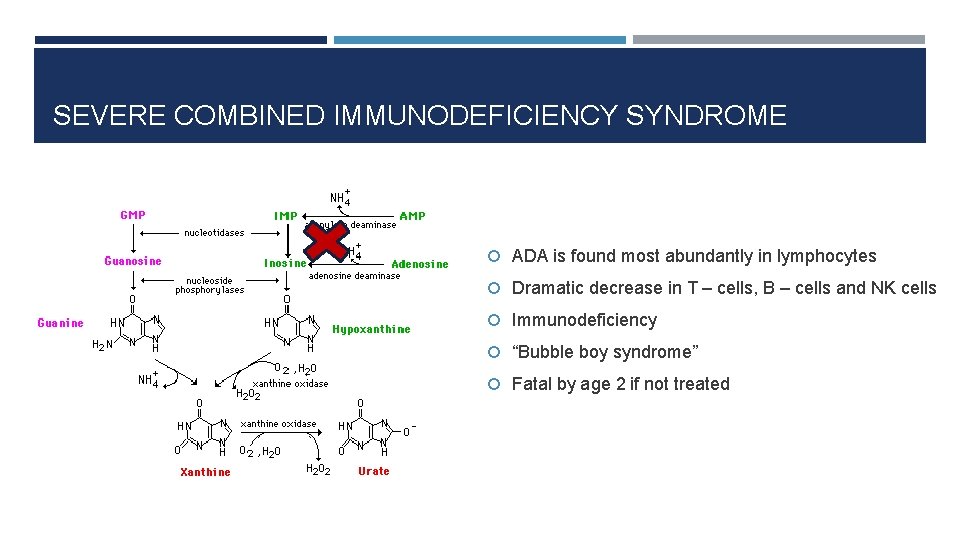
SEVERE COMBINED IMMUNODEFICIENCY SYNDROME ADA is found most abundantly in lymphocytes Dramatic decrease in T – cells, B – cells and NK cells Immunodeficiency “Bubble boy syndrome” Fatal by age 2 if not treated

SCID: TREATMENT Bone marrow transplant Gene therapy ADA coding gene inserted into stem cells and transplanted to patient Enzyme replacement therapy Synthetic ADA in the form of ADA – PEG

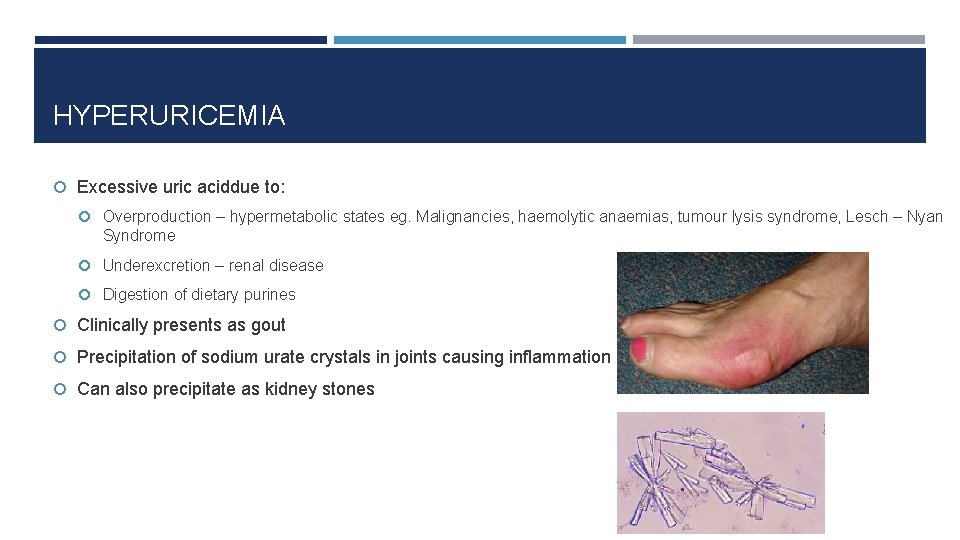
HYPERURICEMIA Excessive uric aciddue to: Overproduction – hypermetabolic states eg. Malignancies, haemolytic anaemias, tumour lysis syndrome, Lesch – Nyan Syndrome Underexcretion – renal disease Digestion of dietary purines Clinically presents as gout Precipitation of sodium urate crystals in joints causing inflammation Can also precipitate as kidney stones

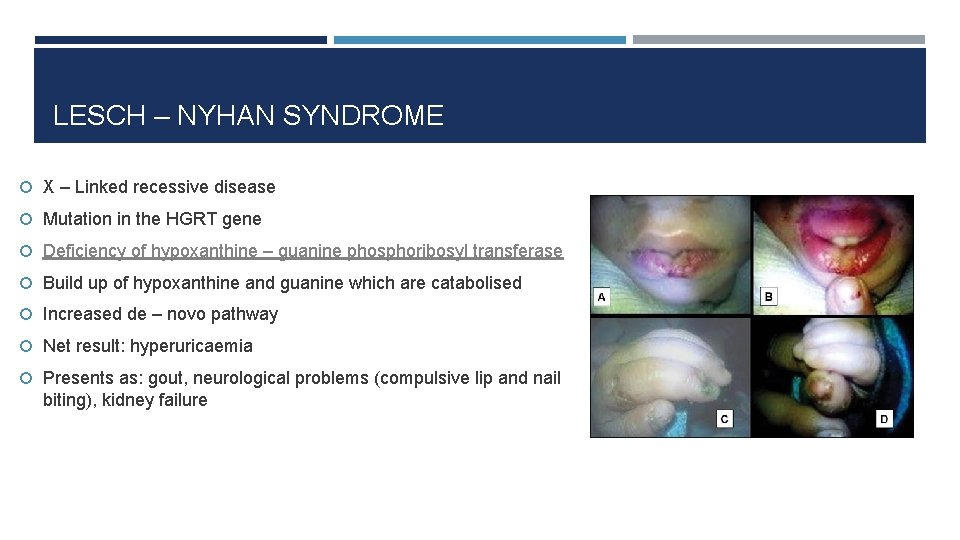
LESCH – NYHAN SYNDROME X – Linked recessive disease Mutation in the HGRT gene Deficiency of hypoxanthine – guanine phosphoribosyl transferase Build up of hypoxanthine and guanine which are catabolised Increased de – novo pathway Net result: hyperuricaemia

PURINE SYNTHESIS: SALVAGE PATHWAY Hypoxanthin e PRPP IMP + PP Adenin e PRPP AMP + 2 P Guanin e PRPP AMP + 2 P Enzyme: hypoxanthine – guanine phosphoribosyl transferase Enzyme: adenine phosphoribosyl transferase Enzyme: hypoxanthine – guanine phosphoribosyl transferase

LESCH – NYHAN SYNDROME X – Linked recessive disease Mutation in the HGRT gene Deficiency of hypoxanthine – guanine phosphoribosyl transferase Build up of hypoxanthine and guanine which are catabolised Increased de – novo pathway Net result: hyperuricaemia Presents as: gout, neurological problems (compulsive lip and nail biting), kidney failure

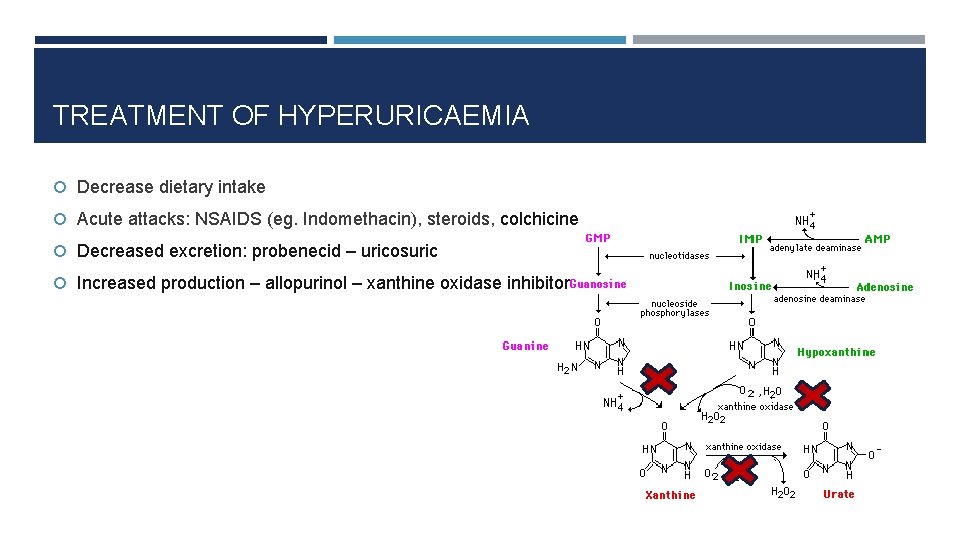
TREATMENT OF HYPERURICAEMIA Decrease dietary intake Acute attacks: NSAIDS (eg. Indomethacin), steroids, colchicine Decreased excretion: probenecid – uricosuric Increased production – allopurinol – xanthine oxidase inhibitor

PYRIMIDINE METABOLISM

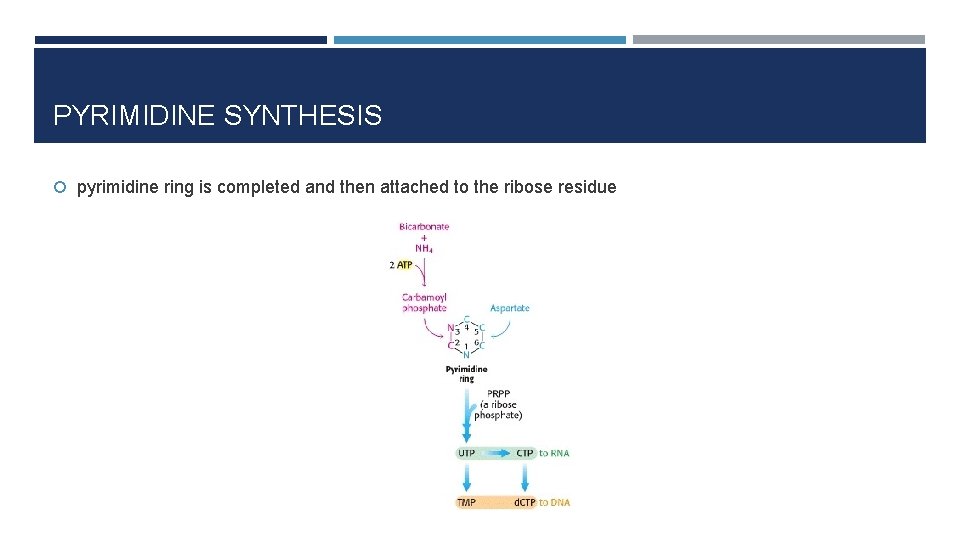
PYRIMIDINE SYNTHESIS pyrimidine ring is completed and then attached to the ribose residue

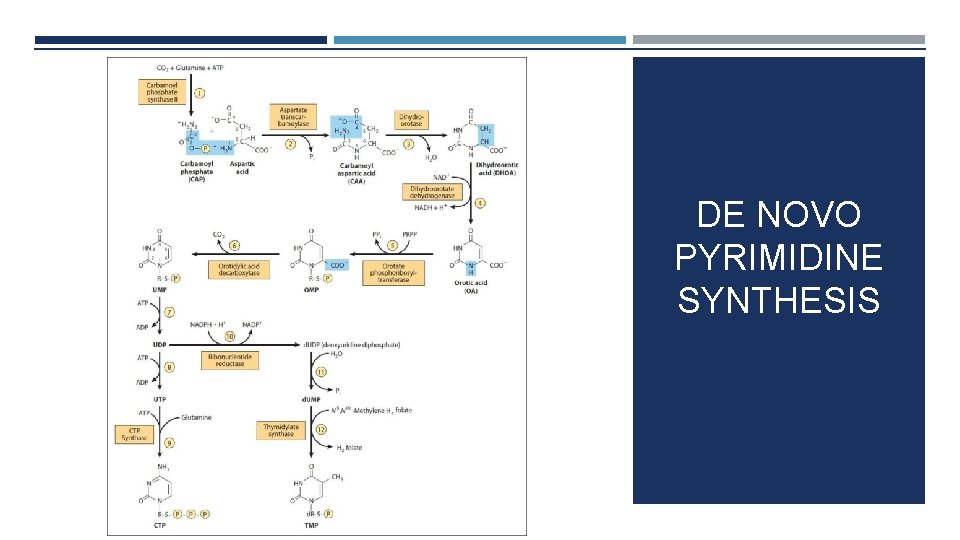
DE NOVO PYRIMIDINE SYNTHESIS

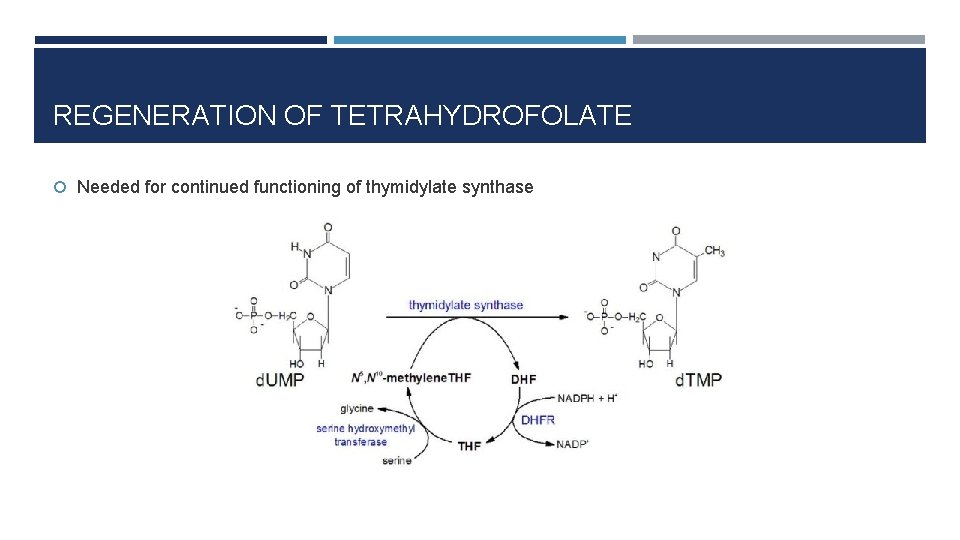
REGENERATION OF TETRAHYDROFOLATE Needed for continued functioning of thymidylate synthase

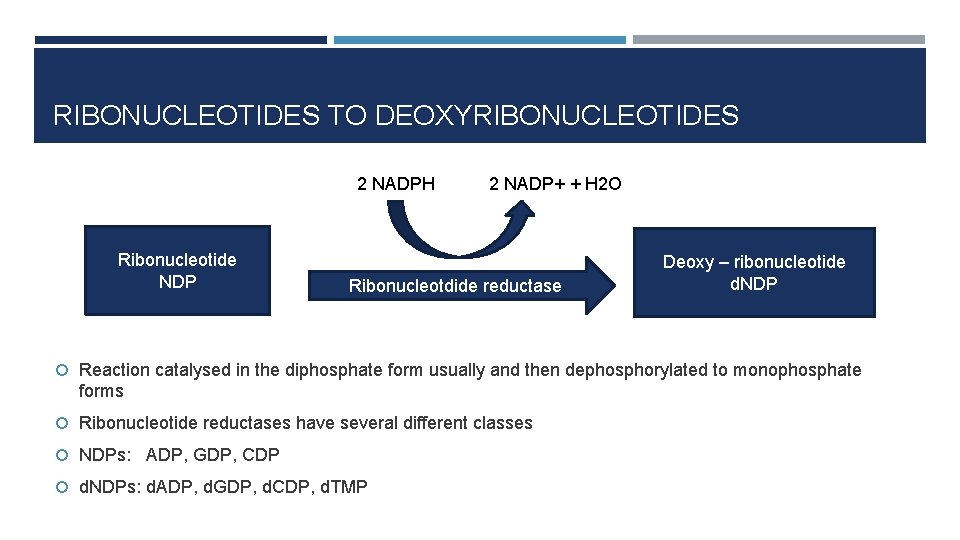
RIBONUCLEOTIDES TO DEOXYRIBONUCLEOTIDES 2 NADPH Ribonucleotide NDP 2 NADP+ + H 2 O Ribonucleotdide reductase Deoxy – ribonucleotide d. NDP Reaction catalysed in the diphosphate form usually and then dephosphorylated to monophosphate forms Ribonucleotide reductases have several different classes NDPs: ADP, GDP, CDP d. NDPs: d. ADP, d. GDP, d. CDP, d. TMP

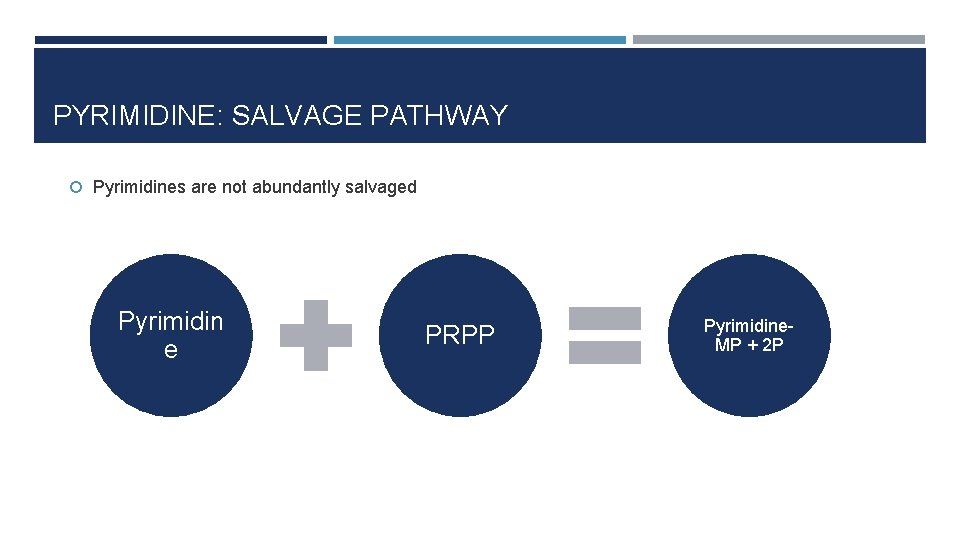
PYRIMIDINE: SALVAGE PATHWAY Pyrimidines are not abundantly salvaged Pyrimidin e PRPP Pyrimidine. MP + 2 P

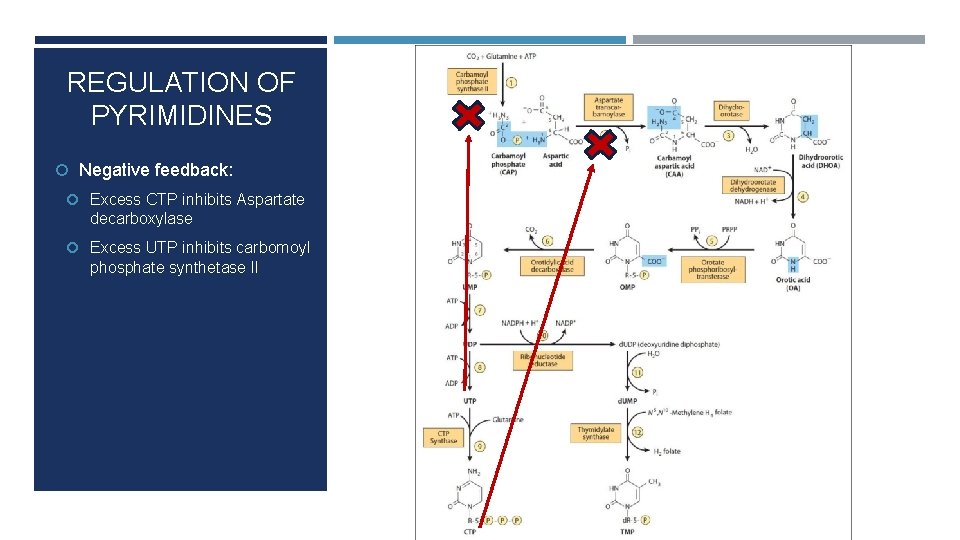
REGULATION OF PYRIMIDINES Negative feedback: Excess CTP inhibits Aspartate decarboxylase Excess UTP inhibits carbomoyl phosphate synthetase II

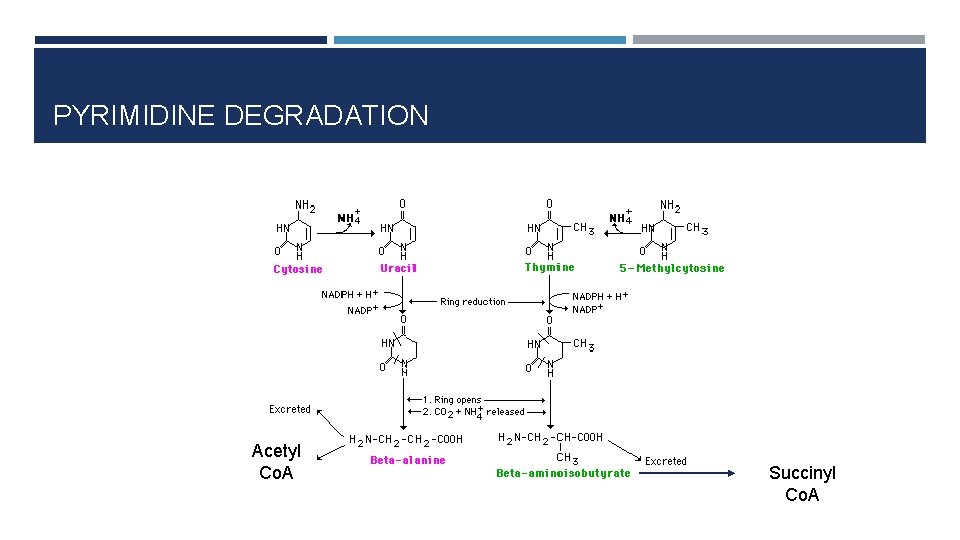
PYRIMIDINE DEGRADATION Acetyl Co. A Succinyl Co. A

CLINICAL SIGNIFICANCE Hereditary Orotic Aciduria Defects in 2 enzymes Presentation: Growth retardation Severe anaemia Orotic acid in urine Patients are given exogenous uridine to simulate salvage pathway

PHARMACOLOGICA L APPLICATIONS

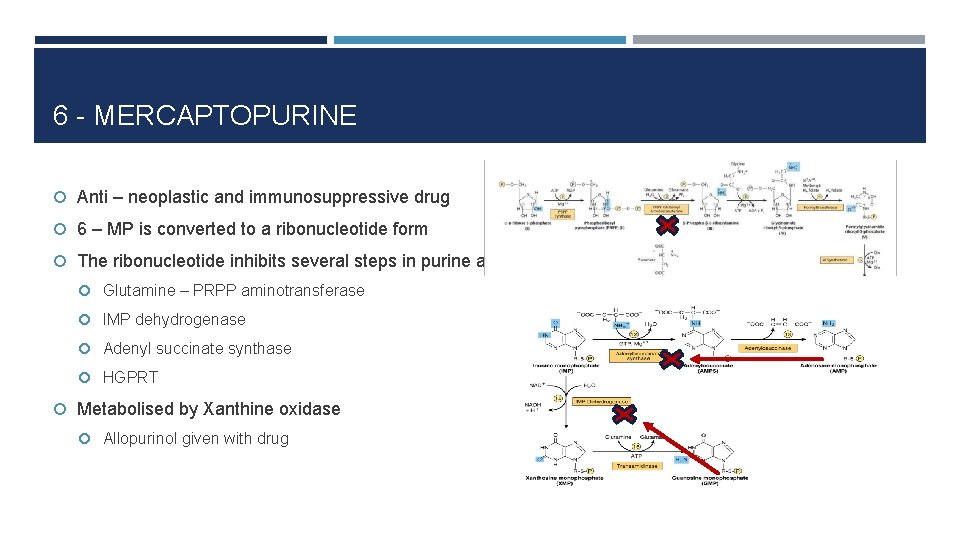
6 - MERCAPTOPURINE Anti – neoplastic and immunosuppressive drug 6 – MP is converted to a ribonucleotide form The ribonucleotide inhibits several steps in purine anabolism: Glutamine – PRPP aminotransferase IMP dehydrogenase Adenyl succinate synthase HGPRT Metabolised by Xanthine oxidase Allopurinol given with drug

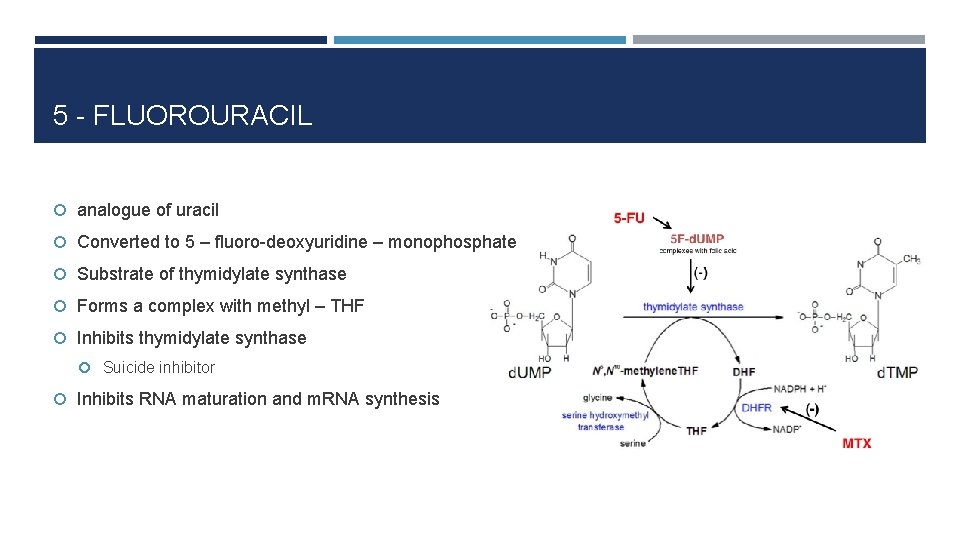
5 - FLUOROURACIL analogue of uracil Converted to 5 – fluoro-deoxyuridine – monophosphate Substrate of thymidylate synthase Forms a complex with methyl – THF Inhibits thymidylate synthase Suicide inhibitor Inhibits RNA maturation and m. RNA synthesis

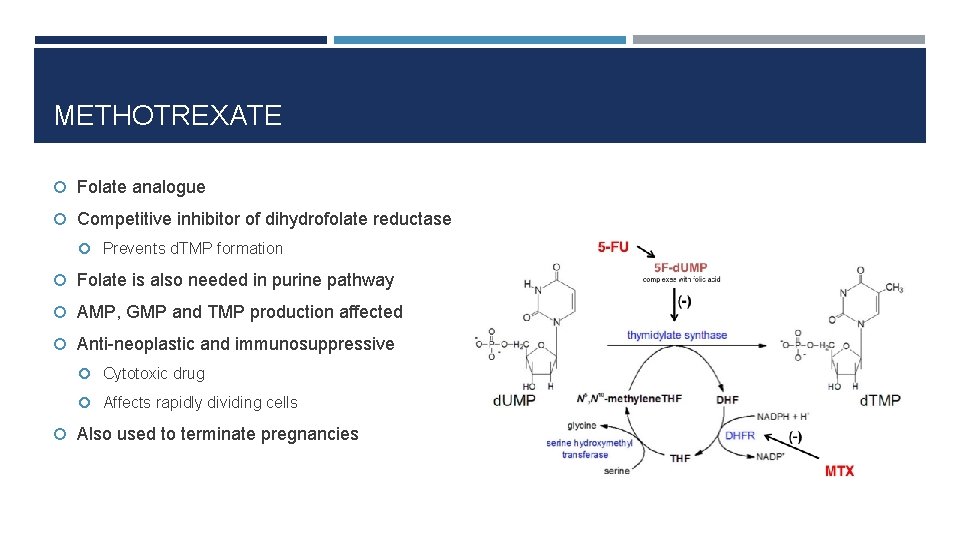
METHOTREXATE Folate analogue Competitive inhibitor of dihydrofolate reductase Prevents d. TMP formation Folate is also needed in purine pathway AMP, GMP and TMP production affected Anti-neoplastic and immunosuppressive Cytotoxic drug Affects rapidly dividing cells Also used to terminate pregnancies

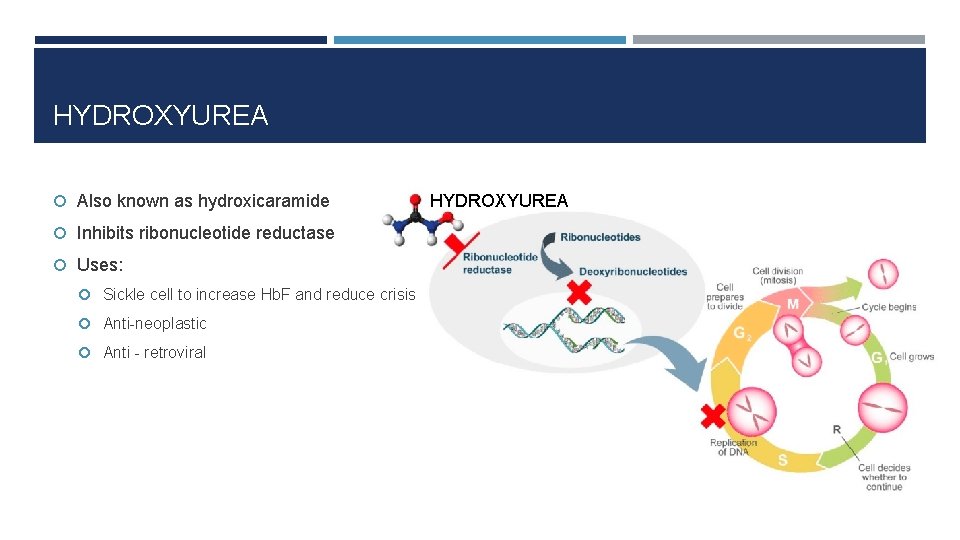
HYDROXYUREA Also known as hydroxicaramide Inhibits ribonucleotide reductase Uses: Sickle cell to increase Hb. F and reduce crisis Anti-neoplastic Anti - retroviral HYDROXYUREA

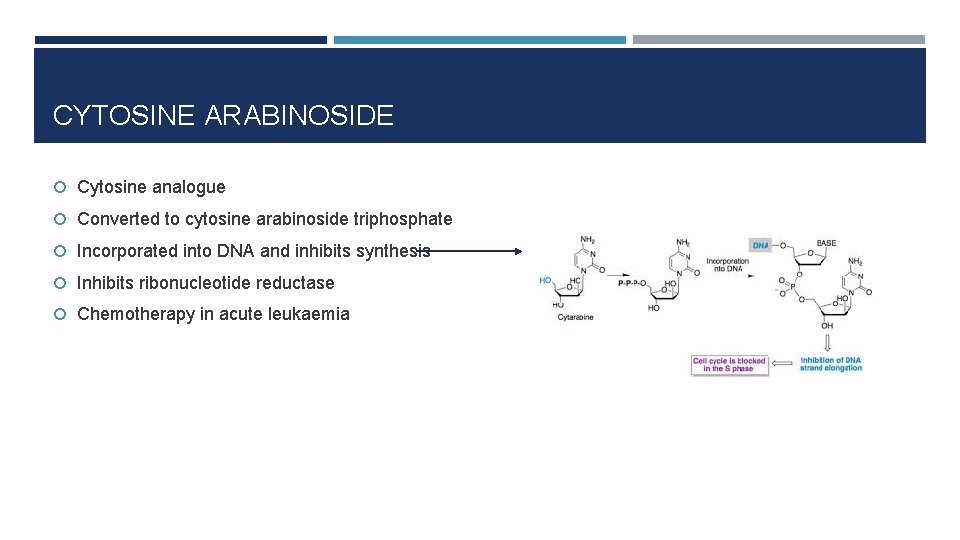
CYTOSINE ARABINOSIDE Cytosine analogue Converted to cytosine arabinoside triphosphate Incorporated into DNA and inhibits synthesis Inhibits ribonucleotide reductase Chemotherapy in acute leukaemia

BASE ANALOGUES AS ANTIVIRALS Acyclovir Guanosine analogue Converted to acylguanosine triphosphate Inhibits DNA polymerase and terminates synthesis Azidothymidine Thymidine analogue Inhibits reverse transcriptase Major drug is HIV treatment

REFERENCES Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5 th edition. New York: W H Freeman; 2002. Section 25. 2, Purine Bases Can Be Synthesized de Novo or Recycled by Salvage Pathways. Available from: https: //www. ncbi. nlm. nih. gov/books/NBK 22385/ https: //basicmedicalkey. com/metabolism-of-purine-pyrimidine-nucleotides/ https: //basicmedicalkey. com/32 -nitrogen-nucleotide-metabolism/
