Introduction to Dispersed Systems FDSC 400 09282001 Goals












- Slides: 22

Introduction to Dispersed Systems FDSC 400 09/28/2001

Goals • • • Scales and Types of Structure in Food Surface Tension Curved Surfaces Surface Active Materials Charged Surfaces

COLLOIDAL SCALE

Dispersed Systems A kinetically stable mixture of one phase in another largely immiscible phase. Usually at least one length scale is in the colloidal range.

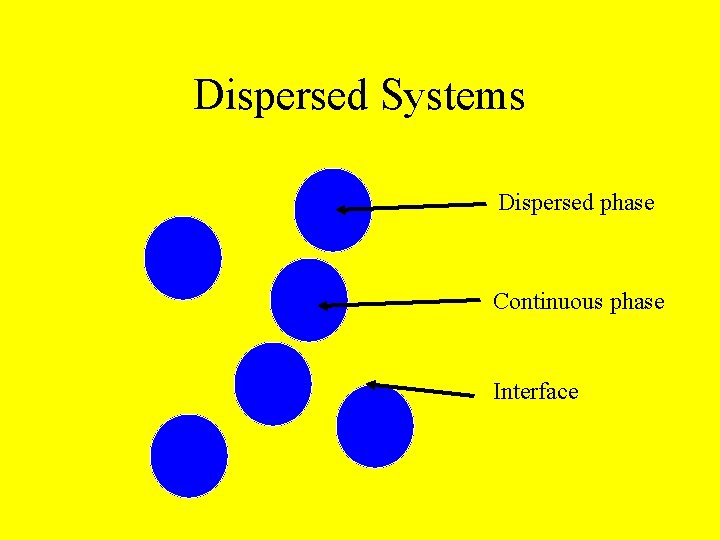
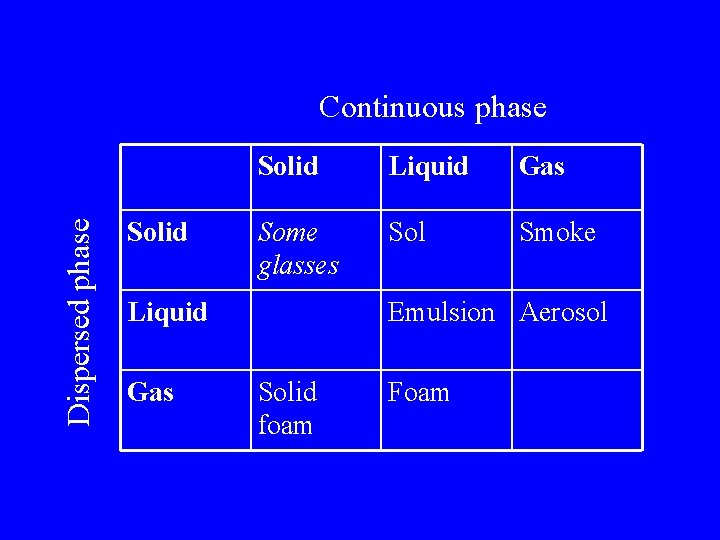
Dispersed Systems Dispersed phase Continuous phase Interface

Dispersed phase Continuous phase Solid Liquid Gas Some glasses Sol Smoke Liquid Gas Emulsion Aerosol Solid foam Foam

Properties of Dispersed Systems • Too small to see • Affected by both gravitational forces and thermal diffusion • Large interfacial area – SURFACE EFFECTS ARE IMPORTANT

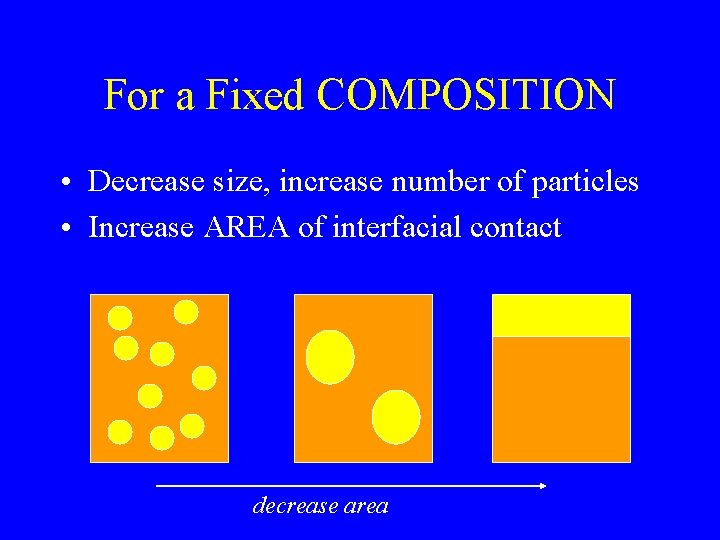
Increased Surface Area We have 20 cm 3 of oil in 1 cm radius droplets. Each has a volume of (4/3. p. r 3) 5. 5 cm 3 and a surface area of (4. p. r 2) 12. 5 cm 2. As we need about 3. 6 droplets we would have a total area of 45. 5 cm 2 The same oil is split into 0. 1 cm radius droplets, each has a volume of 0. 004 cm 3 and a surface area 0. 125 cm 2. As we need about 5000 droplets we would have a total area of 625 cm 2

For a Fixed COMPOSITION • Decrease size, increase number of particles • Increase AREA of interfacial contact decrease area

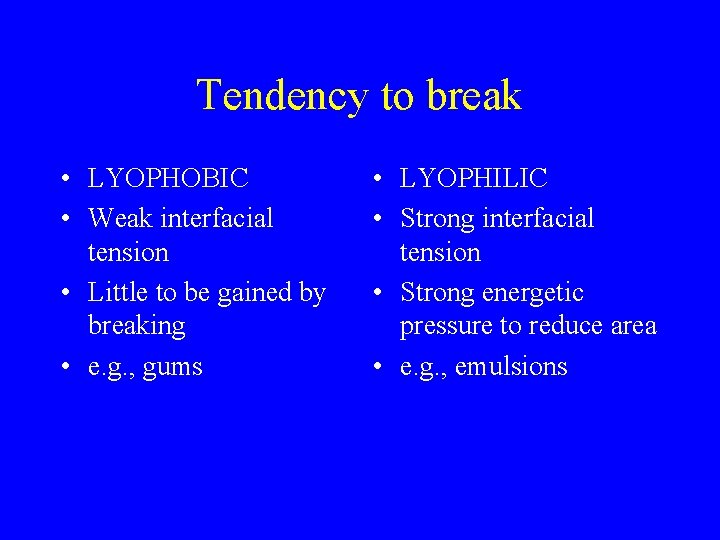
Tendency to break • LYOPHOBIC • Weak interfacial tension • Little to be gained by breaking • e. g. , gums • LYOPHILIC • Strong interfacial tension • Strong energetic pressure to reduce area • e. g. , emulsions

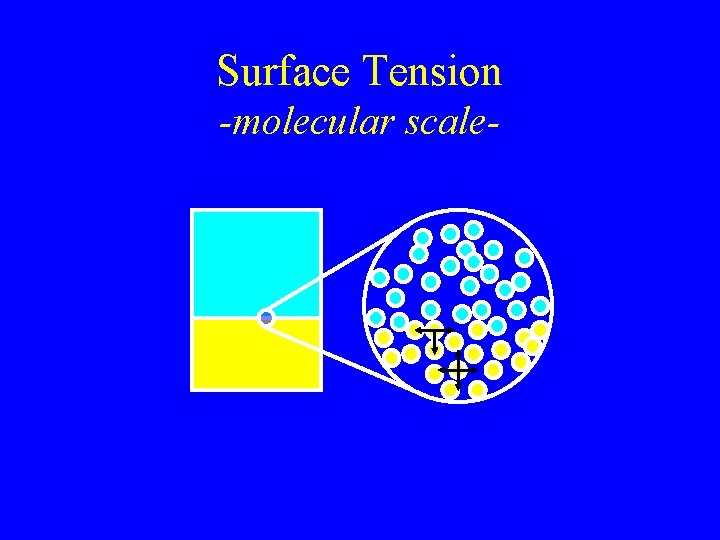
Surface Tension -molecular scale-

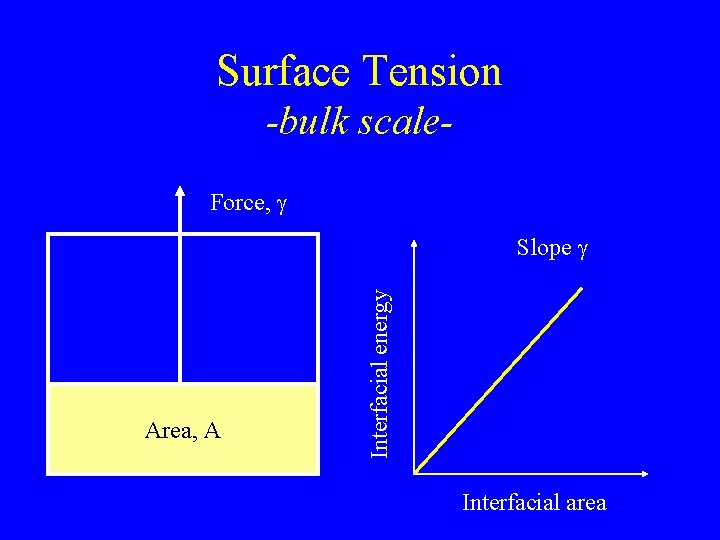
Surface Tension -bulk scale. Force, g Area, A Interfacial energy Slope g Interfacial area

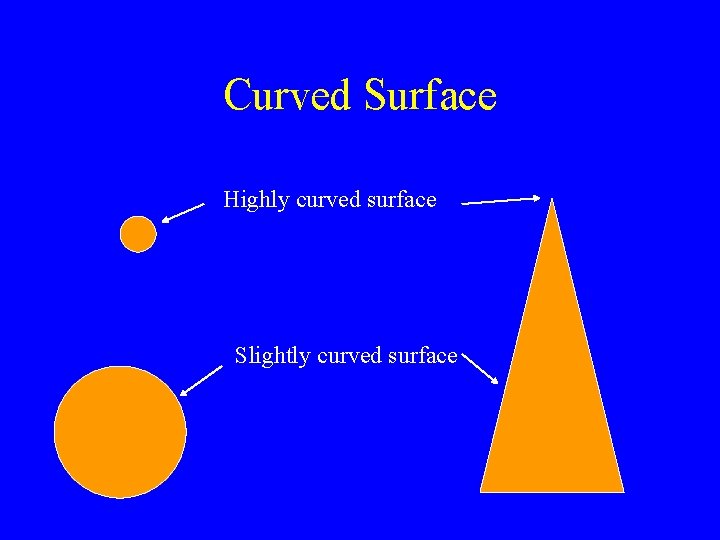
Curved Surface Highly curved surface Slightly curved surface

Curved Surfaces Molecules at highly deformed surfaces are less well anchored into their phase

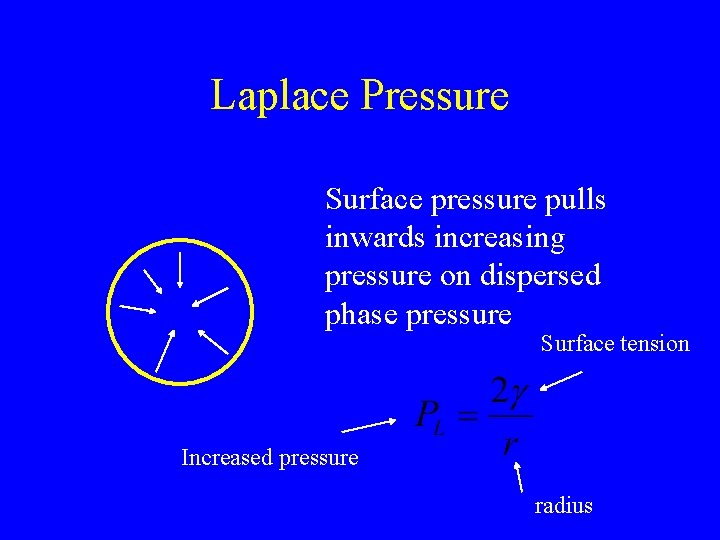
Laplace Pressure Surface pressure pulls inwards increasing pressure on dispersed phase pressure Surface tension Increased pressure radius

Curved Surfaces -Consequences • • Dispersed phase structures tend to be round Small fluid droplets behave as hard spheres Solubility increases with pressure so… Large droplets may grow at the expense of small (Ostwald ripening) – Depends on the solubility of the dispersed phase in the continuous

Surface Active Material • Types of surfactant • Surface accumulation • Surface tension lowering

Types of Surfactant -small molecule. Hydrophilic head group (charged or polar) Hydrophobic tail (non-polar)

Types of Surfactant -polymeric. Polymer backbone Sequence of more water soluble subunits Sequence of less water soluble subunits

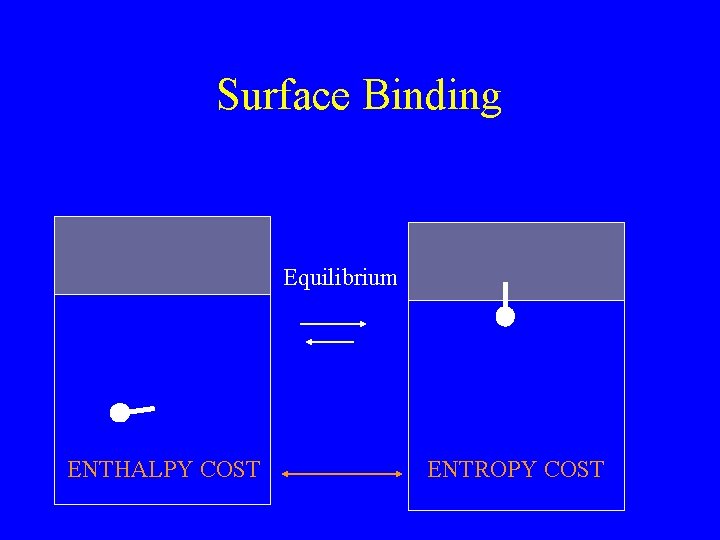
Surface Binding Equilibrium ENTHALPY COST ENTROPY COST

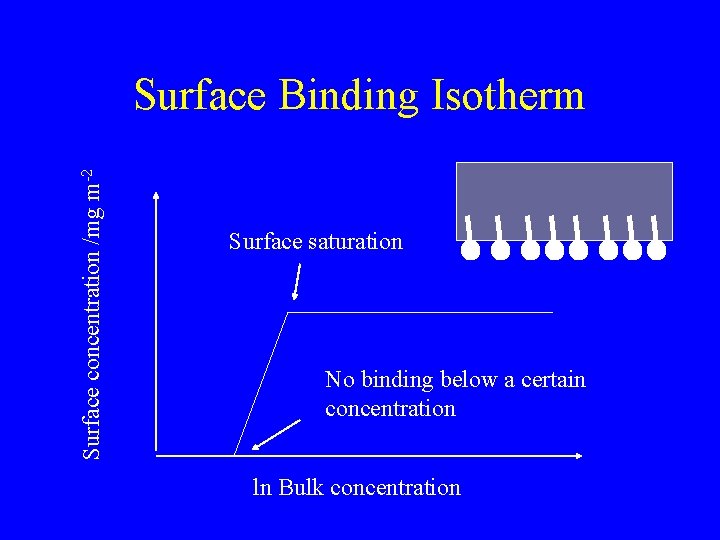
Surface concentration /mg m-2 Surface Binding Isotherm Surface saturation No binding below a certain concentration ln Bulk concentration

Surface Tension Lowering Bare surface (tension g 0) Surface pressure – the ability of a surfactant to lower surface tension Interface partly “hidden” (tension g) p = g-g 0
 400+400+400+200
400+400+400+200 Color 09282001
Color 09282001 Strategic goals tactical goals operational goals
Strategic goals tactical goals operational goals Strategic goals tactical goals operational goals
Strategic goals tactical goals operational goals Ibm geographically dispersed resiliency for power systems
Ibm geographically dispersed resiliency for power systems Iseries high availability
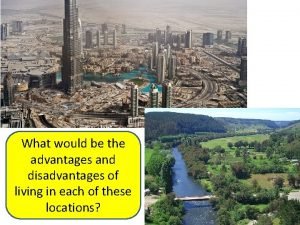
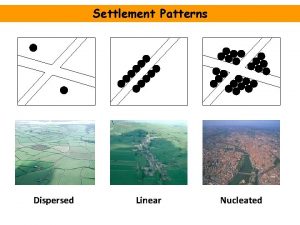
Iseries high availability Nucleated settlement advantages
Nucleated settlement advantages Linear settlement pattern
Linear settlement pattern Nucleated pattern
Nucleated pattern Difference between deflocculated and flocculated suspension
Difference between deflocculated and flocculated suspension Dispersion phase and dispersion medium
Dispersion phase and dispersion medium Interfacial properties of suspended particles ppt
Interfacial properties of suspended particles ppt Isoline map definition ap human geography
Isoline map definition ap human geography Seed splitting
Seed splitting How are bean seeds dispersed
How are bean seeds dispersed Dispersed
Dispersed Parallel sysplex licence charge
Parallel sysplex licence charge Dispersed rural settlement definition
Dispersed rural settlement definition Clustered vs dispersed settlements
Clustered vs dispersed settlements Dispersed phase
Dispersed phase Dispersed collective behavior
Dispersed collective behavior General goals and specific goals
General goals and specific goals Examples of generic goals and product-specific goals
Examples of generic goals and product-specific goals