Introduction to Diamond Blackfan Anemia and Its Treatment

Introduction to Diamond Blackfan Anemia and Its Treatment Adrianna Vlachos, MD The Feinstein Institute for Medical Research Hofstra North Shore-LIJ School of Medicine Cohen Children’s Medical Center of New York DBA Camp July 2015

Diamond Blackfan Anemia n n n Definitions Diagnosis Genetics DBA Registry n Demographics n Congenital Anomalies n Outcomes n Remission n Proven Treatments n Corticosteroids n Transfusion Therapy and Iron Chelation n Stem Cell Transplantation n Experimental Treatments n Leucine n Sotatercept n Cancer Future Directions

DBAR Team n Diamond Blackfan Anemia Registry Adrianna Vlachos, MD n Jeffrey M. Lipton, MD, Ph. D n Eva Atsidaftos, MA n Jessica Kang, BS n n 1 -888 -884 -DBAR

DBA SAP Team n DBA Surveillance and Awareness Program Adrianna Vlachos, MD n Jeffrey M. Lipton, MD, Ph. D n Johnson Liu, MD - Medical Hematology n Sandeep Jauhar, MD – Cardiology n Yael Toby Harris, MD – Endocrinology n Phyllis Speiser, MD – Pediatric Endocrinology n

Acknowledgements n n n n DBA patients, their families, and their physicians Diamond Blackfan Anemia Foundation Daniella Maria Arturi Foundation Pediatric Cancer Foundation NHLBI (R 01 and Resequencing Project) CDC DOD Our numerous collaborators

Collaborators n n National Human Genome Research Institute/NIH n David Bodine, Ph. D n Kelly O’Brien University of Arkansas n Jason Farrar, MD University of Louisville n Steven R Ellis, Ph. D Phoenix Children’s Hospital n Robert Arceci, MD, Ph. D+ n n National Cancer Institute/NIH n Blanche Alter, MD, MPH n Philip Rosenberg, Ph. D Children’s Hospital Boston n Hanna Gazda, MD n Alan Beggs, Ph. D Children’s Hospital of Philadelphia n Monica Bessler, MD, Ph. D St Mary’s Hospital, UK n Josu De La Fuente, MD n Sarah Ball, MD

DBA is an Inherited Bone Marrow Failure Syndrome (IBMFS) IBMFS have Key Shared Characteristics: n Pathophysiology Mutant cells have a low threshold for apoptosis Apoptosis = programmed cell death n n Clinical n n Bone Marrow Failure Congenital Anomalies Cancer Predisposition May present in adulthood
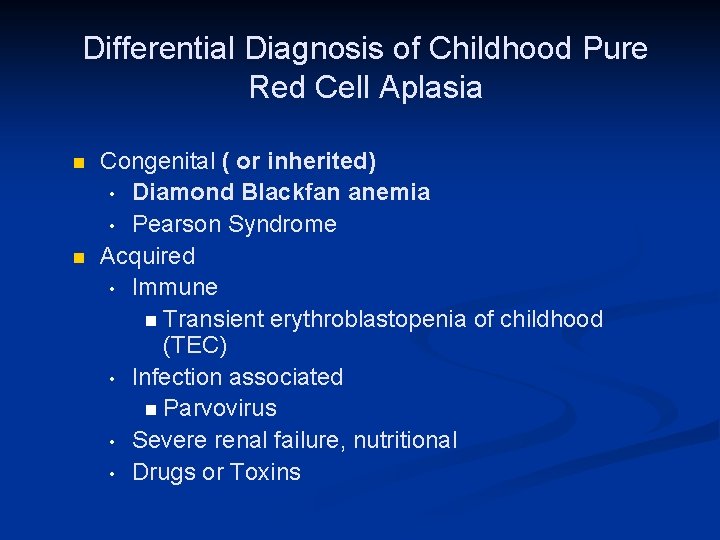
Differential Diagnosis of Childhood Pure Red Cell Aplasia n n Congenital ( or inherited) • Diamond Blackfan anemia • Pearson Syndrome Acquired • Immune n Transient erythroblastopenia of childhood (TEC) • Infection associated n Parvovirus • Severe renal failure, nutritional • Drugs or Toxins

Expanded Definition of DBA n Results of International Registries n n n More Robust Epidemiology = the science that studies the patterns, causes, and effects of health and disease conditions in defined populations. Gene Discovery Discoveries n Ten of 14 published “DBA genes” discovered through the DBAR: n n Demonstrate extremely variable expression within and between families Demonstrate that DBA is not rarely inherited - but is familial with autosomal dominant transmission in almost 50% of cases (RPS 19, Sarah Ball)

Diamond Blackfan Anemia “Classic” Diagnostic Criteria n Described by Josephs in 1936 and Diamond and Blackfan in 1938 as a ‘pure’ red cell aplasia n “Classic” Definition in 1976 by Alter and colleagues: n n Moderate to severe macrocytic anemia Reticulocytopenia Normal bone marrow cellularity with a scarcity of red cell precursors Age less than 1 year
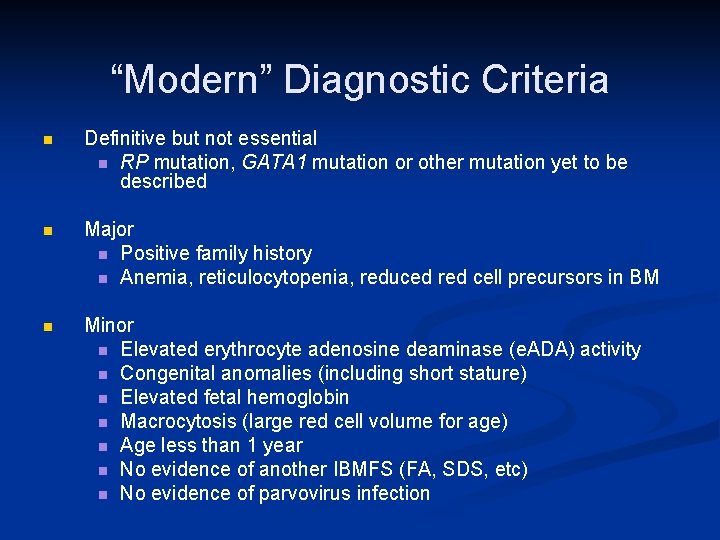
“Modern” Diagnostic Criteria n Definitive but not essential n RP mutation, GATA 1 mutation or other mutation yet to be described n Major n Positive family history n Anemia, reticulocytopenia, reduced red cell precursors in BM n Minor n Elevated erythrocyte adenosine deaminase (e. ADA) activity n Congenital anomalies (including short stature) n Elevated fetal hemoglobin n Macrocytosis (large red cell volume for age) n Age less than 1 year n No evidence of another IBMFS (FA, SDS, etc) n No evidence of parvovirus infection

Criteria for an Expanded Diagnosis “non-classic DBA” A patient meeting some of the classic criteria and having a known DBA-associated mutation A patient with a positive family history and no features of DBA and having a known DBA-associated mutation “probable DBA” Some major and some minor criteria
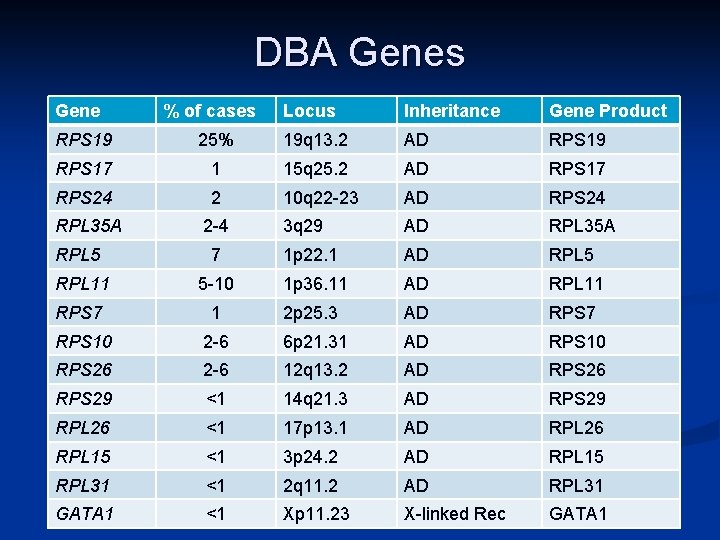
DBA Genes Gene % of cases Locus Inheritance Gene Product RPS 19 25% 19 q 13. 2 AD RPS 19 RPS 17 1 15 q 25. 2 AD RPS 17 RPS 24 2 10 q 22 -23 AD RPS 24 RPL 35 A 2 -4 3 q 29 AD RPL 35 A RPL 5 7 1 p 22. 1 AD RPL 5 RPL 11 5 -10 1 p 36. 11 AD RPL 11 RPS 7 1 2 p 25. 3 AD RPS 7 RPS 10 2 -6 6 p 21. 31 AD RPS 10 RPS 26 2 -6 12 q 13. 2 AD RPS 26 RPS 29 <1 14 q 21. 3 AD RPS 29 RPL 26 <1 17 p 13. 1 AD RPL 26 RPL 15 <1 3 p 24. 2 AD RPL 15 RPL 31 <1 2 q 11. 2 AD RPL 31 GATA 1 <1 Xp 11. 23 X-linked Rec GATA 1
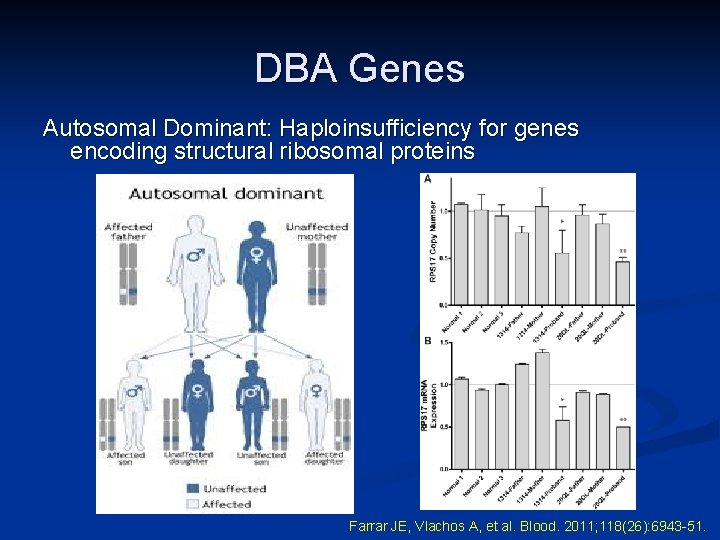
DBA Genes Autosomal Dominant: Haploinsufficiency for genes encoding structural ribosomal proteins Farrar JE, Vlachos A, et al. Blood. 2011; 118(26): 6943 -51.

Diamond Blackfan Anemia Registry (DBAR) n The DBAR of North America was formally established in 1991 n Today the DBAR is a robust tool for studying DBA

Objective of the DBAR n To develop a demographic, clinical and laboratory database in order to facilitate the study of n the epidemiology of DBA n the biology of DBA
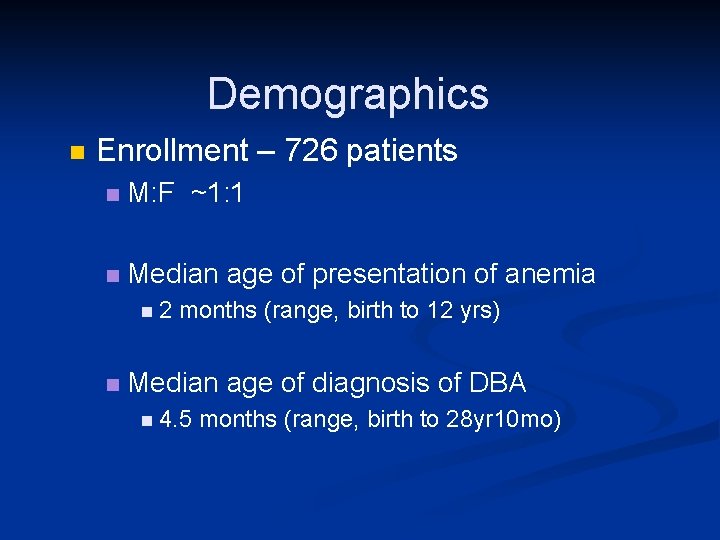
Demographics n Enrollment – 726 patients n M: F ~1: 1 n Median age of presentation of anemia n 2 months (range, birth to 12 yrs) n Median age of diagnosis of DBA n 4. 5 months (range, birth to 28 yr 10 mo)
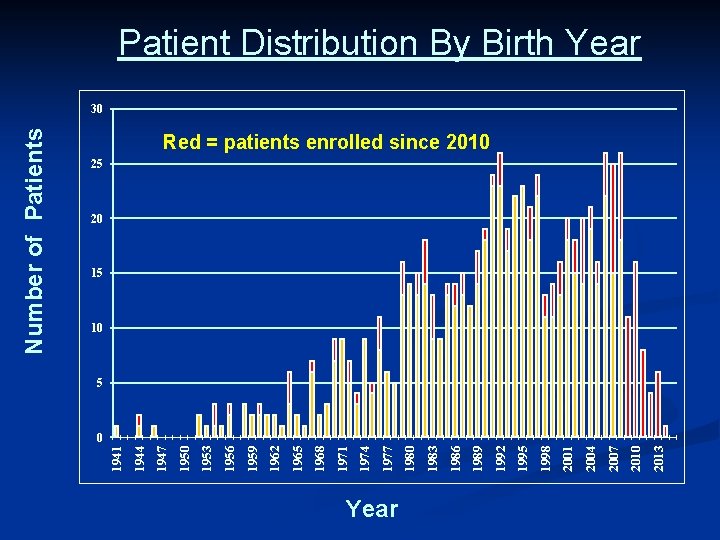
Year 2013 2010 2007 2004 2001 1998 1995 1992 1989 1986 1983 1980 1977 1974 1971 1968 1965 1962 1959 1956 1953 1950 1947 1944 1941 Number of Patients Patient Distribution By Birth Year 30 Red = patients enrolled since 2010 25 20 15 10 5 0
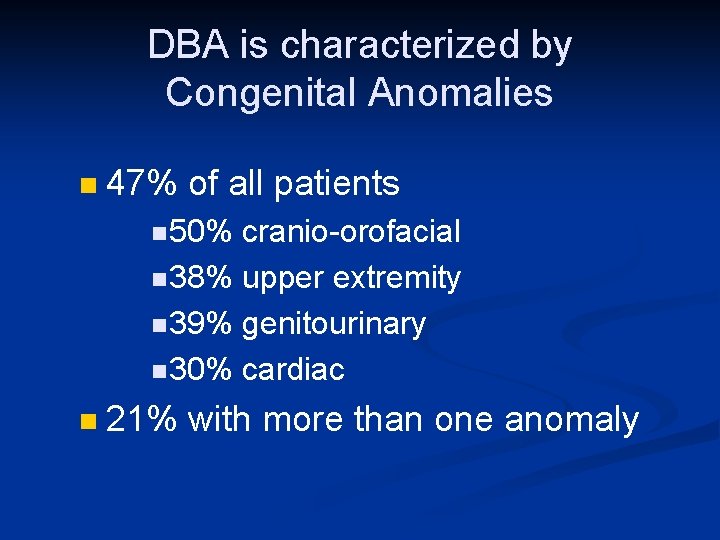
DBA is characterized by Congenital Anomalies n 47% of all patients n 50% cranio-orofacial n 38% upper extremity n 39% genitourinary n 30% cardiac n 21% with more than one anomaly

Cleft Palates in DBA n Confounding diagnoses • Treacher Collins syndrome • Pierre Robin sequence

DBA Patient Reported with Treacher Collins Syndrome n n Absent lower eyelashes Deformed ears Small cheek bones Short, recessed chin
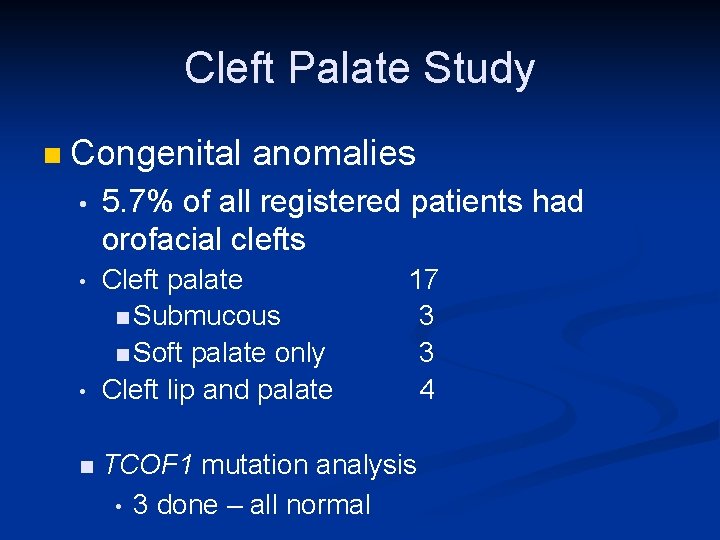
Cleft Palate Study n Congenital anomalies • 5. 7% of all registered patients had orofacial clefts • Cleft palate 17 n Submucous 3 n Soft palate only 3 Cleft lip and palate 4 • n TCOF 1 mutation analysis • 3 done – all normal
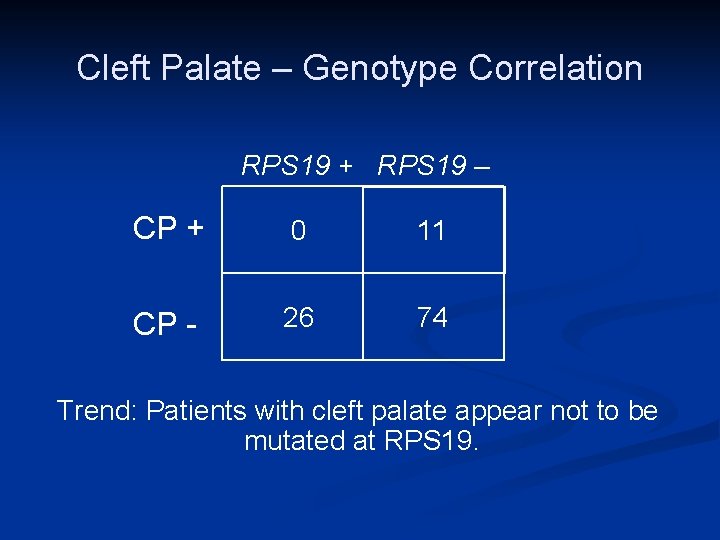
Cleft Palate – Genotype Correlation RPS 19 + RPS 19 – CP + 0 CP - 11 26 74 Trend: Patients with cleft palate appear not to be mutated at RPS 19.
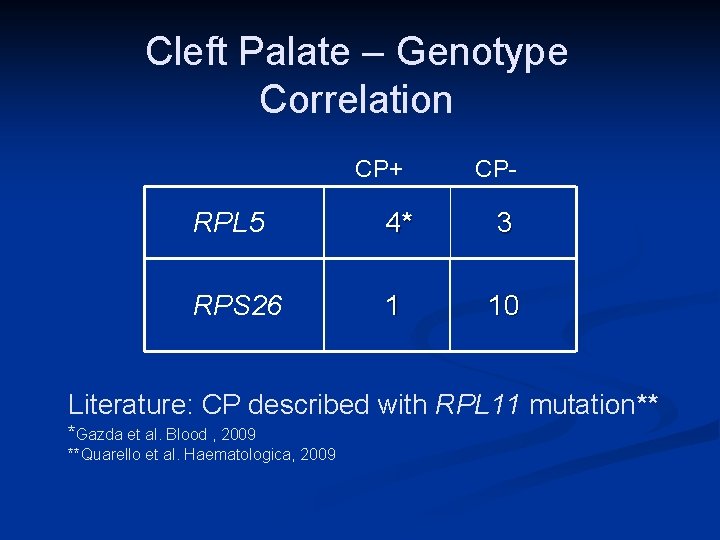
Cleft Palate – Genotype Correlation CP+ CP- RPL 5 4* 3 RPS 26 1 10 Literature: CP described with RPL 11 mutation** *Gazda et al. Blood , 2009 **Quarello et al. Haematologica, 2009

Conclusions from this study n n n DBA patients with orofacial clefting represent a “family” of distinct DBA genotypes Mutations in RPL 5 and RPS 26 (and RPL 11) are associated with cleft palate/lip Helps in genetic screening as well
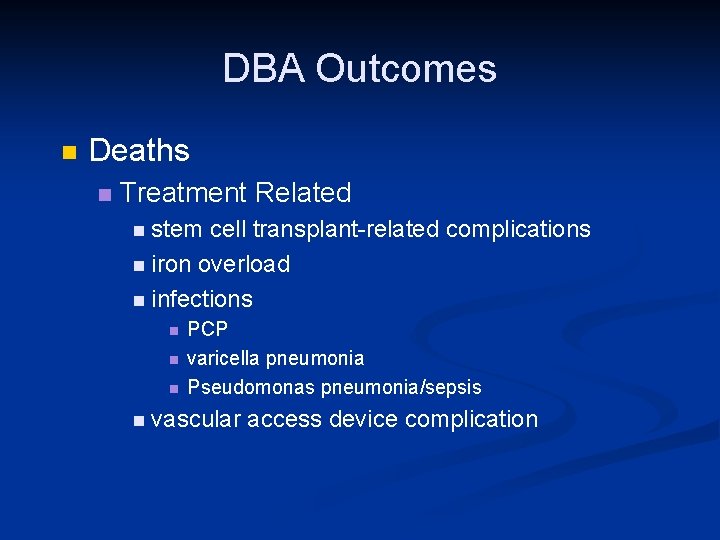
DBA Outcomes n Deaths n Treatment Related n stem cell transplant-related complications n iron overload n infections n n n PCP varicella pneumonia Pseudomonas pneumonia/sepsis n vascular access device complication
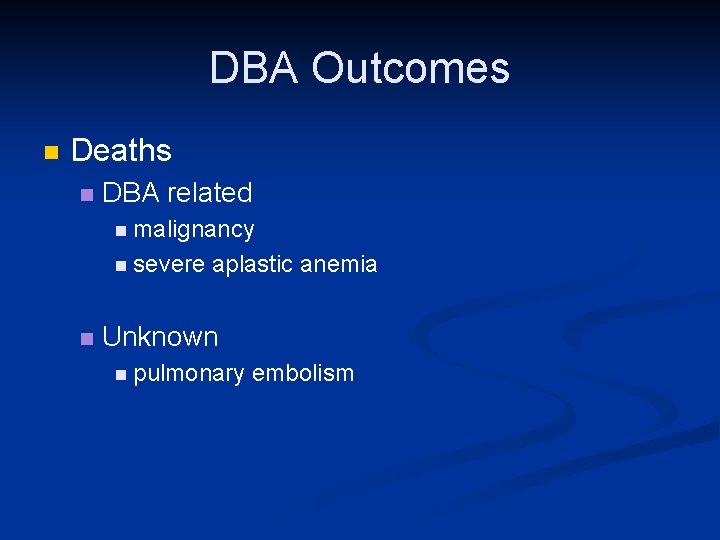
DBA Outcomes n Deaths n DBA related n malignancy n severe aplastic anemia n Unknown n pulmonary embolism
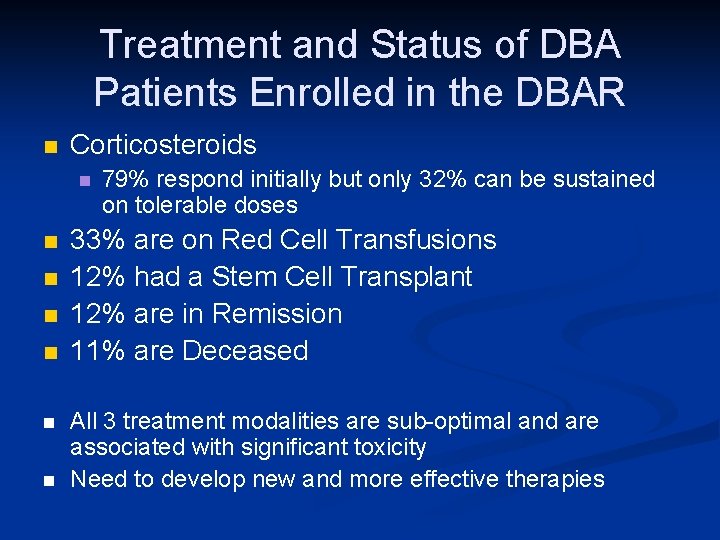
Treatment and Status of DBA Patients Enrolled in the DBAR n Corticosteroids n n n n 79% respond initially but only 32% can be sustained on tolerable doses 33% are on Red Cell Transfusions 12% had a Stem Cell Transplant 12% are in Remission 11% are Deceased All 3 treatment modalities are sub-optimal and are associated with significant toxicity Need to develop new and more effective therapies

Remission n 568 patients enrolled in the DBAR 79 patients experienced a remission 71 patients were available for analysis Same 1: 1 Male: female ratio as DBAR n Median age at diagnosis: 3 mo n

Remission Results n n n 73% entered remission while on steroids 16% in remission while receiving both steroids and transfusions 8% in remission from chronic transfusions

Remission Results n Median age of remission for all pts: 5. 7 years (range, 0. 3 to 46. 6 years) males n females n n 5. 8 years (range, 0. 3 to 46. 6) 4. 8 years (range, 0. 9 to 26) Median duration of treatment to remission: 38 months (range, 1 month to 37. 6 years)

Remission Results n Median duration of remission: 11. 5 years (range, 6 months to 48. 1 years) n The actuarial likelihood of entering remission is approximately 20% by 25 years of age
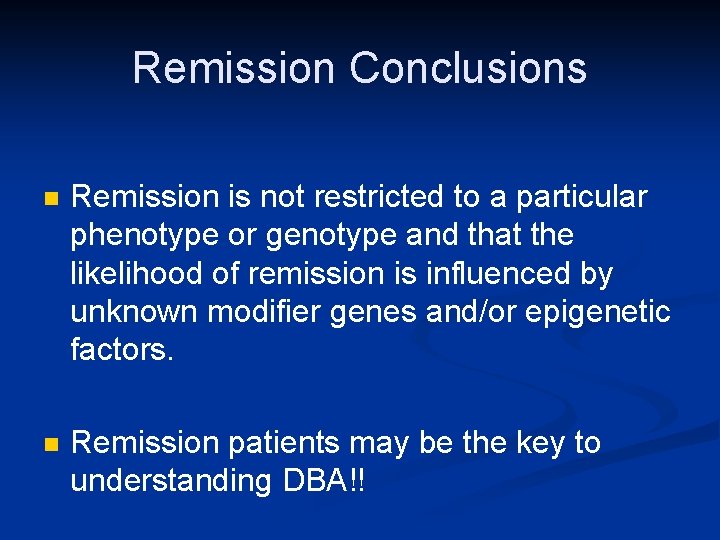
Remission Conclusions n Remission is not restricted to a particular phenotype or genotype and that the likelihood of remission is influenced by unknown modifier genes and/or epigenetic factors. n Remission patients may be the key to understanding DBA!!

Treatments for DBA n Transfusion therapy n Chronic red cell transfusion regimen n Starts when Hb is less than 8 gm/d. L n Transfuse 10 -15 ml/kg every 3 -4 weeks n Goal: maintain adequate quality of life while maintaining growth n Ideally transfuse until vaccinations given (age 1) n May need to end earlier if venous access difficulty n If needs Port, please ask for a plastic one
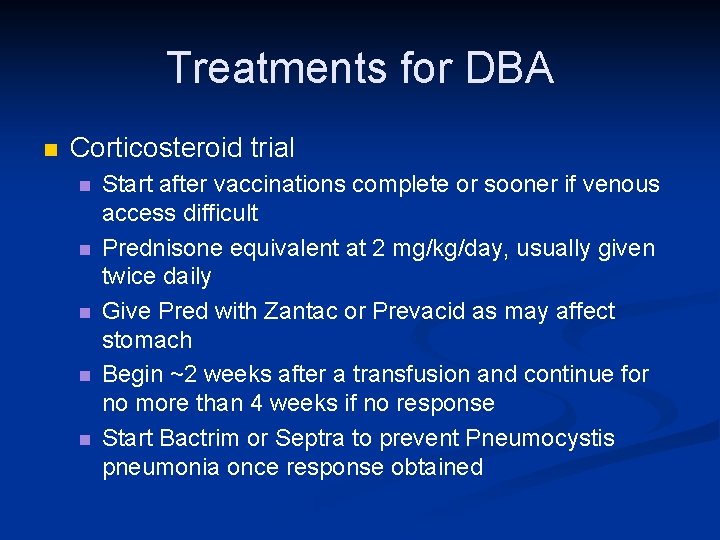
Treatments for DBA n Corticosteroid trial n n n Start after vaccinations complete or sooner if venous access difficult Prednisone equivalent at 2 mg/kg/day, usually given twice daily Give Pred with Zantac or Prevacid as may affect stomach Begin ~2 weeks after a transfusion and continue for no more than 4 weeks if no response Start Bactrim or Septra to prevent Pneumocystis pneumonia once response obtained

Treatments for DBA n Corticosteroid therapy If response noted than wean to 1 mg/kg/day over 2 months and then 0. 5 mg/kg/day or 1 mg/kg/every other day Goal: Best response at the lowest dose possible n May need 3 x/week or 2 x/week n Must be brave enough to wean, as patient might be in remission n

Treatments for DBA n Corticosteroid therapy Watch growth!!!! n If falling off growth curve, or having pathologic fractures, need to consider a steroid hiatus n n Consult endocrinology for all patients, sooner if having growth issues

Treatments for DBA If no response to steroids then discontinue!! – do NOT increase dose n Resume transfusion therapy n Maintain growth and keep Hb>8 or higher n At 10 -15 transfusions, need to begin chelation therapy n n Deferoxamine (Desferal) – SC or IV n Deferasirox (Exjade) - PO n Deferasirox (Jadenu) - PO n Deferiprone (Ferriprox) - PO

Treatments for DBA n Chelation therapy n After age 2, begin n n n Exjade at 20 -40 mg/kg/day Jadenu at 14 -28 mg/kg/day If ferritin not decreasing, may need to consider Desferal therapy Once able, obtain T 2* to check heart and liver iron load Consider liver biopsy if high If iron overload high, start Desferal at 50 -60 mg/kg/day over 10 -12 hours/day subcutaneously

Treatments for DBA n Chelation therapy n n n If too high or cardiac issues, need to give Desferal 24 hrs/day intravenously Can combine Exjade/Jadenu and Desferal Ferriprox may cause low white cell counts (neutropenia) and has caused severe infection and death But, is a very good drug for unloading iron stored in the heart So, is used in patients in cardiac failure with very strict follow-up
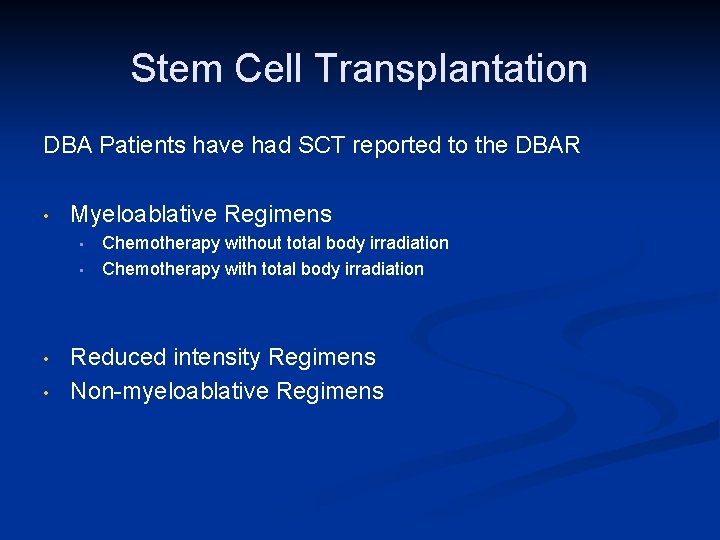
Stem Cell Transplantation DBA Patients have had SCT reported to the DBAR • Myeloablative Regimens • • Chemotherapy without total body irradiation Chemotherapy with total body irradiation Reduced intensity Regimens Non-myeloablative Regimens
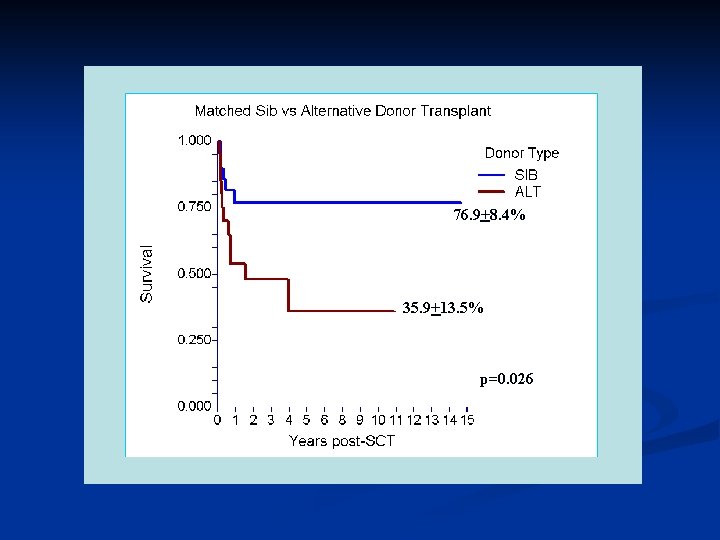
76. 9+8. 4% 35. 9+13. 5% p=0. 026
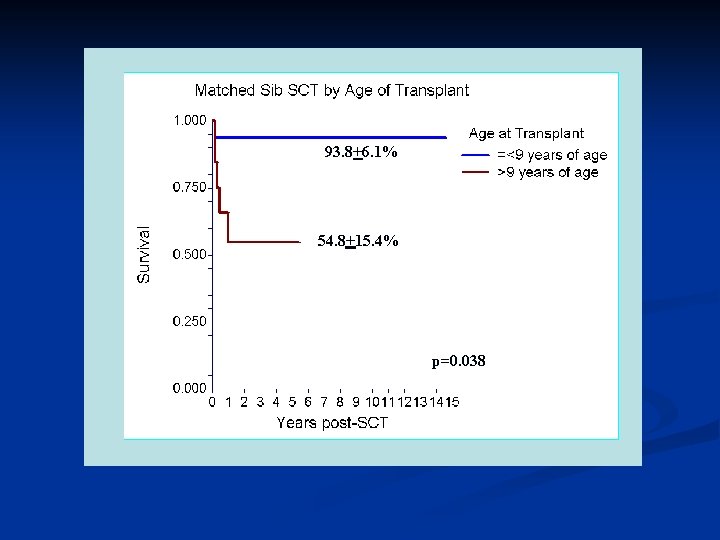
93. 8+6. 1% 54. 8+15. 4% p=0. 038
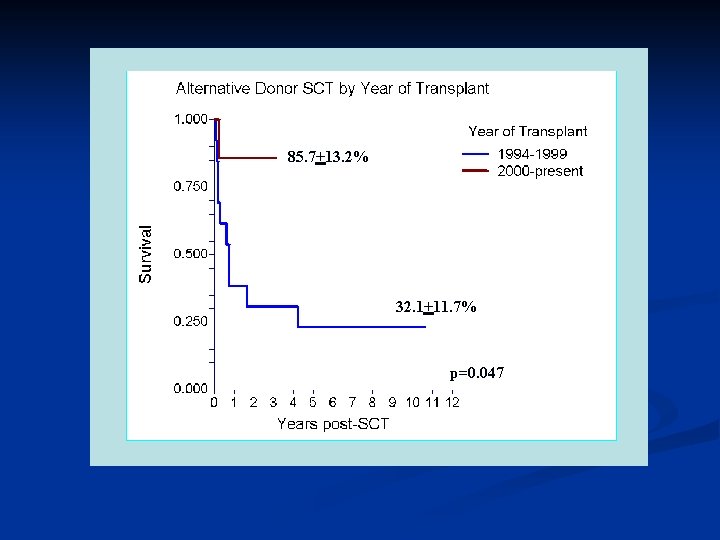
85. 7+13. 2% 32. 1+11. 7% p=0. 047
Outcomes Stem Cell Transplantation n Causes of death Infection n Veno-occlusive disease (VOD) n Graft vs Host Disease (Gv. HD) n Graft failure/rejection n Secondary cancers n

Characteristics of Cancer in DBA n n n Hematologic malignancies Young age at diagnosis Poor prognosis
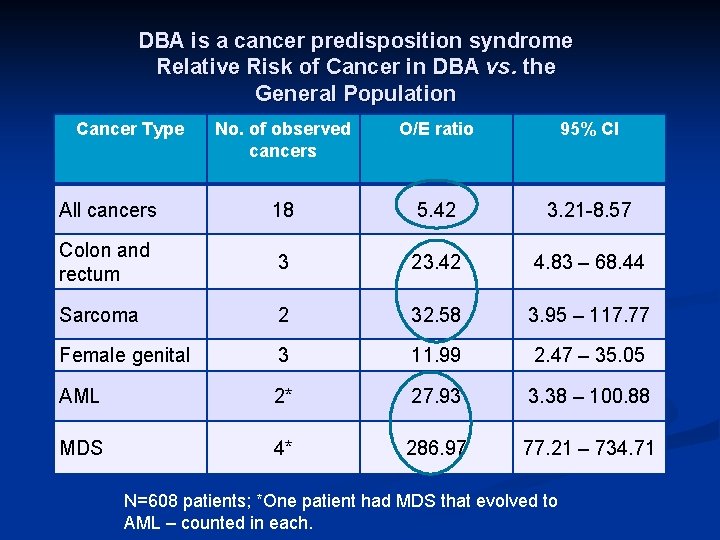
DBA is a cancer predisposition syndrome Relative Risk of Cancer in DBA vs. the General Population Cancer Type No. of observed cancers O/E ratio 95% CI All cancers 18 5. 42 3. 21 -8. 57 Colon and rectum 3 23. 42 4. 83 – 68. 44 Sarcoma 2 32. 58 3. 95 – 117. 77 Female genital 3 11. 99 2. 47 – 35. 05 AML 2* 27. 93 3. 38 – 100. 88 MDS 4* 286. 97 77. 21 – 734. 71 N=608 patients; *One patient had MDS that evolved to AML – counted in each.
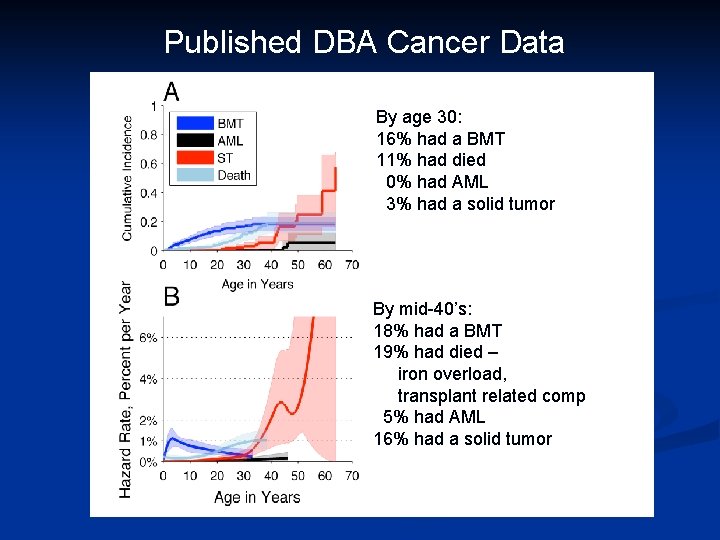
Published DBA Cancer Data By age 30: 16% had a BMT 11% had died 0% had AML 3% had a solid tumor By mid-40’s: 18% had a BMT 19% had died – iron overload, transplant related comp 5% had AML 16% had a solid tumor
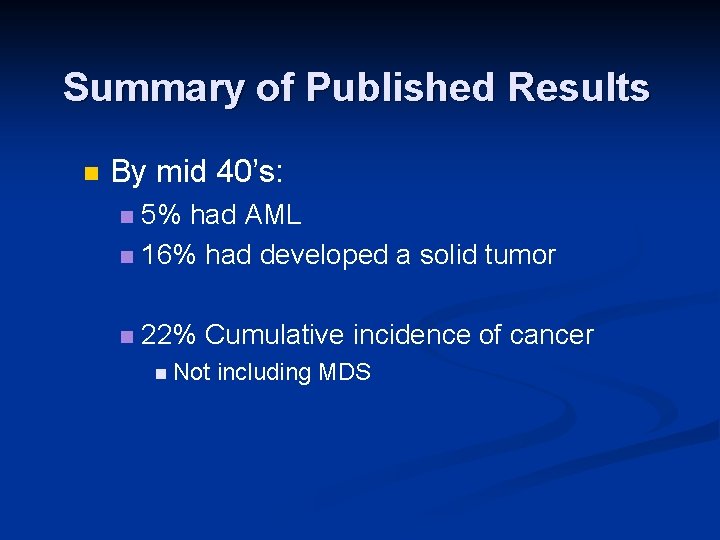
Summary of Published Results n By mid 40’s: 5% had AML n 16% had developed a solid tumor n n 22% Cumulative incidence of cancer n Not including MDS

Neoplasms in DBA Patients from the DBAR n n n n Gastrointestinal Cancers Osteogenic Sarcoma/Other Sarcoma Gynecological Cancer Skin Cancer Hematologic malignancies MDS Median age at presentation of 1 st cancer: 41 years (2 -69)
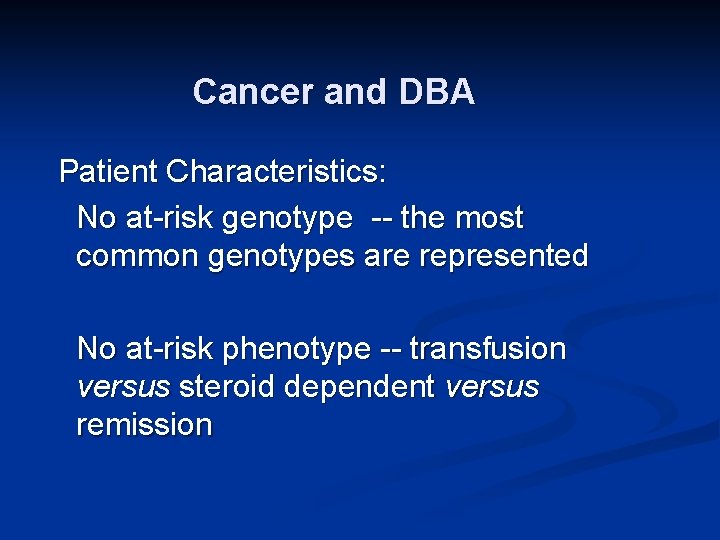
Cancer and DBA Patient Characteristics: No at-risk genotype -- the most common genotypes are represented No at-risk phenotype -- transfusion versus steroid dependent versus remission
Cancer and DBA Initial Patient Characteristics n transfusion dependent at time of cancer n steroid dependent n never received treatment n were in remission n was 4 years status post BMT n was 15 years status post BMT

Cancer Outcomes n n Some patients with cures Deaths due to neutropenia with chemotherapy, leading to sepsis Deaths leading to progressive disease due to not getting treatments as per schedule (because of low counts) IMPROVING – with EARLY diagnosis and individualized treatments
Cancer Outcomes n EARLY diagnosis Screening for cancer n More solid tumors than leukemia, but may be more MDS n n INDIVIDUALIZED treatments Use of Neupogen for low neutrophil counts n Possible dose reduction of chemotherapy when warranted n

Future Directions n n n Continue gene discovery – still with 25% of patients to be diagnosed – with Dr. Bodine Report stem cell transplant results Redo cancer analysis – with Dr. Alter and Dr. Rosenberg Continue clinical trials Continue to inform medical hematologists about DBA!!!
- Slides: 55