Introduction to Chemistry Section 1 Section 2 Section












- Slides: 30

Introduction to Chemistry Section 1: Section 2: Section 3: Section 4: A Story of Two Substances Chemistry and Matter Scientific Methods Scientific Research

• Section 1: Chemistry is the study of everything around us. • Section 2: Branches of chemistry involve the study of different kinds of matter. • Section 3: Scientists use scientific methods to systematically pose and test solutions to questions and assess the results of the tests. • Section 4: Some scientific investigations result in the development of technology that can improve our lives and the world around us.

Vocabulary Review • • matter technology systematic approach synthetic New • • chemistry substance mass weight model scientific method qualitative data New continued • • • quantitative data hypothesis experiment independent variable control conclusion theory scientific law pure research applied research

A Story of Two Substances Section 1

Section 1: A Story of Two Substances • Chemistry is the study of everything around us. K What I Know W What I Want to Find Out L What I Learned

Why Study Chemistry? • All the “stuff” in the universe is made from building blocks formed in stars. • These building blocks and everything made from them are called _______. • ______is the study of matter and the changes it undergoes.

The Ozone Layer • Ultraviolet _____ damages living organisms. • Earth’s atmosphere contains a layer of _______ that absorbs most ultraviolet radiation and protects living organisms. • Ozone is a substance in the atmosphere made up of ______. • A ______, also known as a chemical, is matter that has a definite composition.

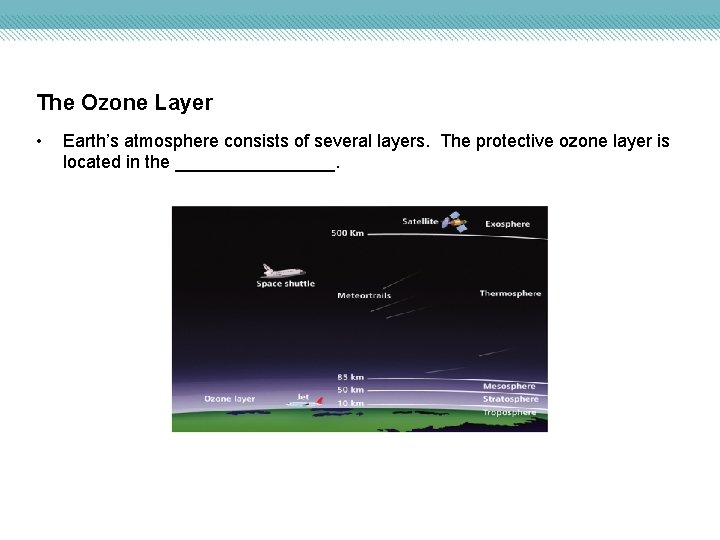
The Ozone Layer • Earth’s atmosphere consists of several layers. The protective ozone layer is located in the ________.

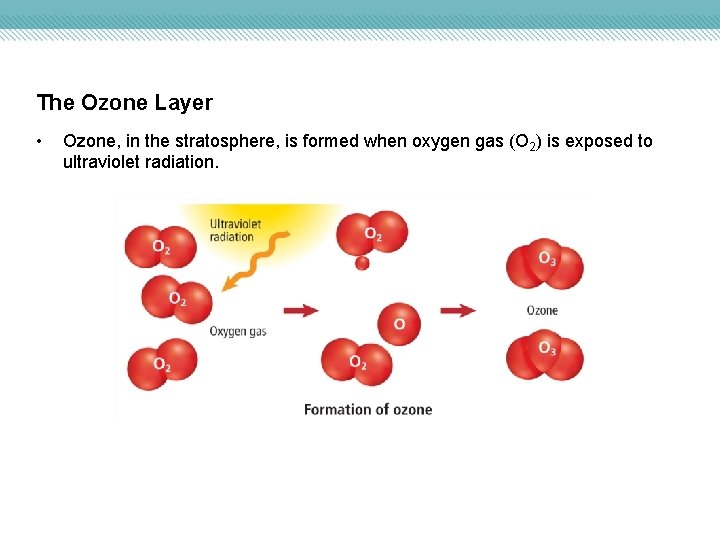
The Ozone Layer • Ozone, in the stratosphere, is formed when oxygen gas (O 2) is exposed to ultraviolet radiation.

The Ozone Layer • Ozone has interested and been studied by scientists since the late 1800’s. • Ozone forms over the ____, where the rays of sunlight are the strongest and then flows towards the poles, thus, making it a convenient marker to follow the flow of air in the stratosphere. The Ozone Layer

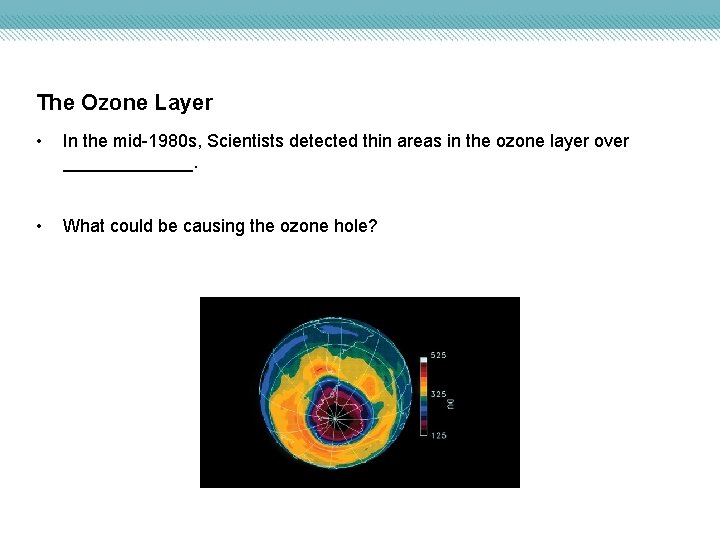
The Ozone Layer • In the mid-1980 s, Scientists detected thin areas in the ozone layer over _______. • What could be causing the ozone hole?

Chlorofluorocarbons • In the 1920’s, large-scale production of refrigerators began, which used ammonia as coolant. • In an attempt to find safer coolant, chemist, Thomas Midgley, Jr. synthesized the first chlorofluorocarbons in 1928. • A Chlorofluorocarbon (CFC) is a substance that consists of _____, _______and _____. • All substances that are classified as CFCs are: – Man-made (they do not occur naturally) – Nontoxic – Stable (they do not readily react with other substances) • Because of being nontoxic and very _____, they seemed to be ideal coolants for refrigerators and AC units, for use in plastic foams and as propellants in spray cans.

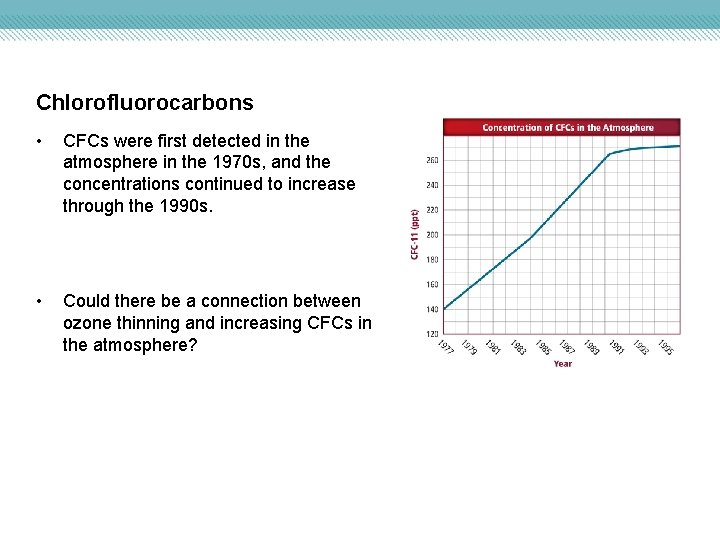
Chlorofluorocarbons • CFCs were first detected in the atmosphere in the 1970 s, and the concentrations continued to increase through the 1990 s. • Could there be a connection between ozone thinning and increasing CFCs in the atmosphere?

Review Essential Questions • What is a substance? • How does ozone form and why is it important? • What are chlorofluorocarbons and how do they get into the atmosphere? Vocabulary • chemistry • substance

Chemistry and Matter Section 2

Section 2: Chemistry and Matter • Branches of chemistry involve the study of different kinds of matter. K What I Know W What I Want to Find Out L What I Learned

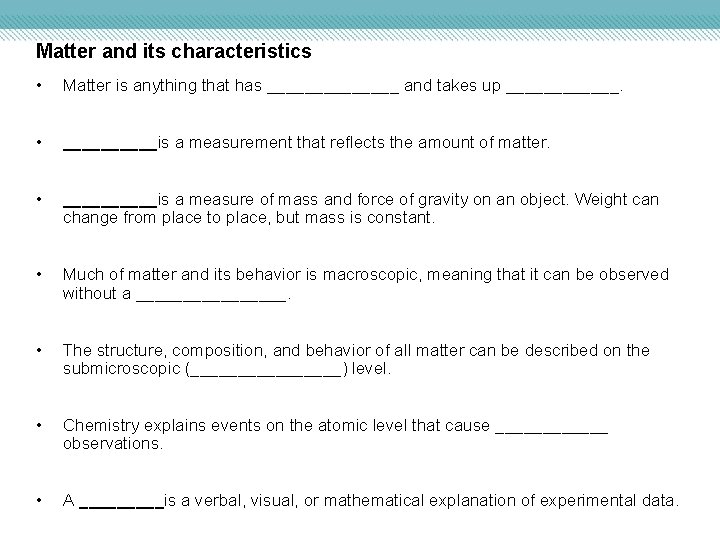
Matter and its characteristics • Matter is anything that has _______ and takes up ______. • _____is a measurement that reflects the amount of matter. • _____is a measure of mass and force of gravity on an object. Weight can change from place to place, but mass is constant. • Much of matter and its behavior is macroscopic, meaning that it can be observed without a ________. • The structure, composition, and behavior of all matter can be described on the submicroscopic (________) level. • Chemistry explains events on the atomic level that cause ______ observations. • A _____is a verbal, visual, or mathematical explanation of experimental data.

Chemistry: The Central Science • A basic understanding of chemistry is central to all sciences – biology, physics, Earth science, ecology, etc. Chemistry is traditionally broken into ________that focus on specific areas such as: – Organic chemistry – Inorganic chemistry – Physical chemistry – Analytical chemistry – Biochemistry – Environmental chemistry – Industrial chemistry – Polymer chemistry – Theoretical chemistry – thermochemistry

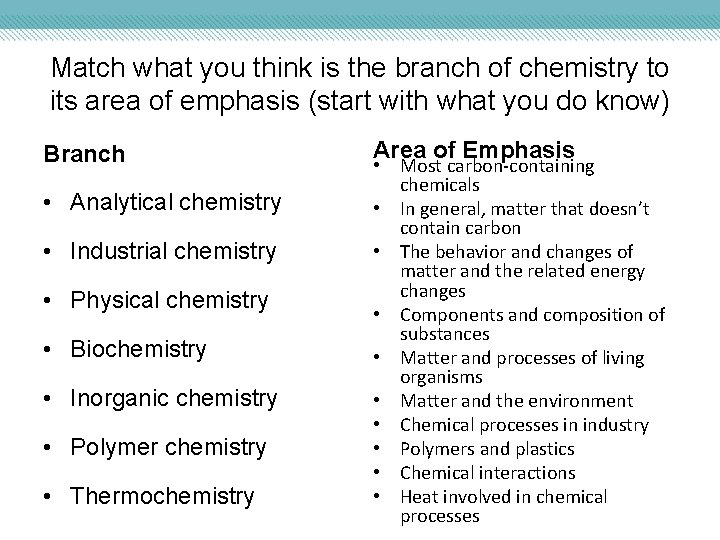
Match what you think is the branch of chemistry to its area of emphasis (start with what you do know) Branch • Analytical chemistry • Industrial chemistry • Physical chemistry • Biochemistry • Inorganic chemistry • Polymer chemistry • Thermochemistry Area of Emphasis • Most carbon-containing chemicals • In general, matter that doesn’t contain carbon • The behavior and changes of matter and the related energy changes • Components and composition of substances • Matter and processes of living organisms • Matter and the environment • Chemical processes in industry • Polymers and plastics • Chemical interactions • Heat involved in chemical processes

Review Essential Questions • How do mass and weight compare and contrast? • Why are chemists interested in a submicroscopic description of matter? • What defines the various branches of chemistry? Vocabulary • Mass • weight • model

Scientific Methods & Research Section 3 & Section 4

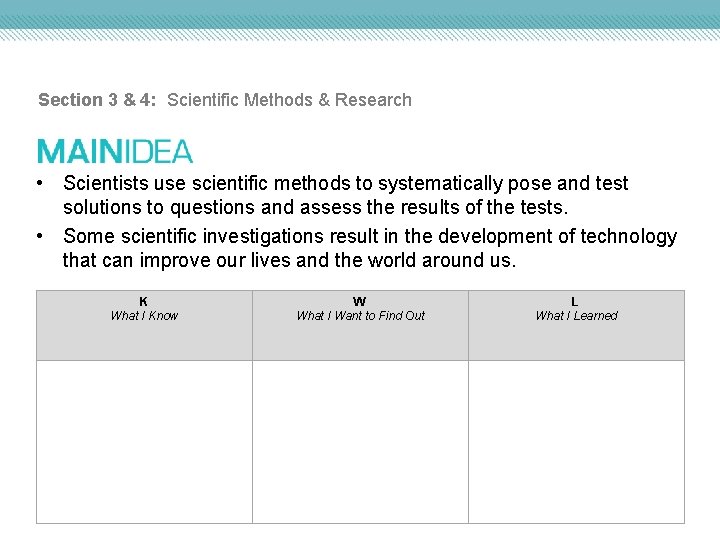
Section 3 & 4: Scientific Methods & Research • Scientists use scientific methods to systematically pose and test solutions to questions and assess the results of the tests. • Some scientific investigations result in the development of technology that can improve our lives and the world around us. K What I Know W What I Want to Find Out L What I Learned

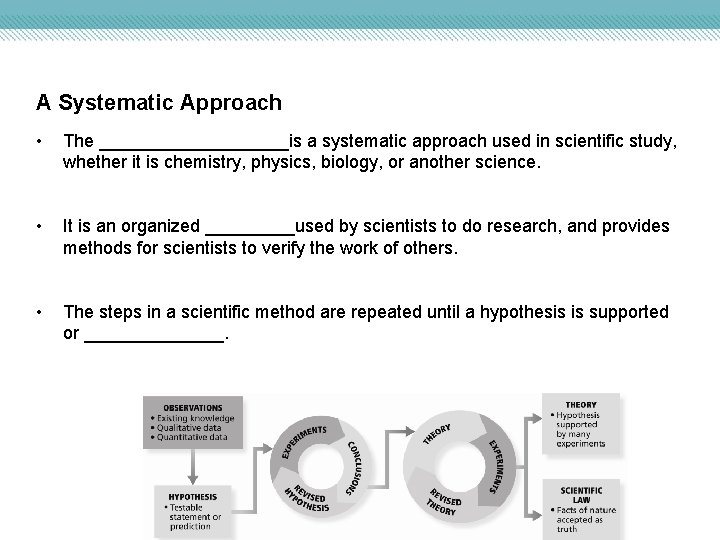
A Systematic Approach • The __________is a systematic approach used in scientific study, whether it is chemistry, physics, biology, or another science. • It is an organized _____used by scientists to do research, and provides methods for scientists to verify the work of others. • The steps in a scientific method are repeated until a hypothesis is supported or _______.

A Systematic Approach • An observation is the act of gathering information. – _______is obtained through observations that describe color, smell, shape, or some other physical characteristic that is related to the 5 senses. – ________is obtained from numerical observations that describe how much, how little, how big, or how fast. • A ________is a tentative explanation for what has been observed. • An _______is a set of controlled observations that test the hypothesis.

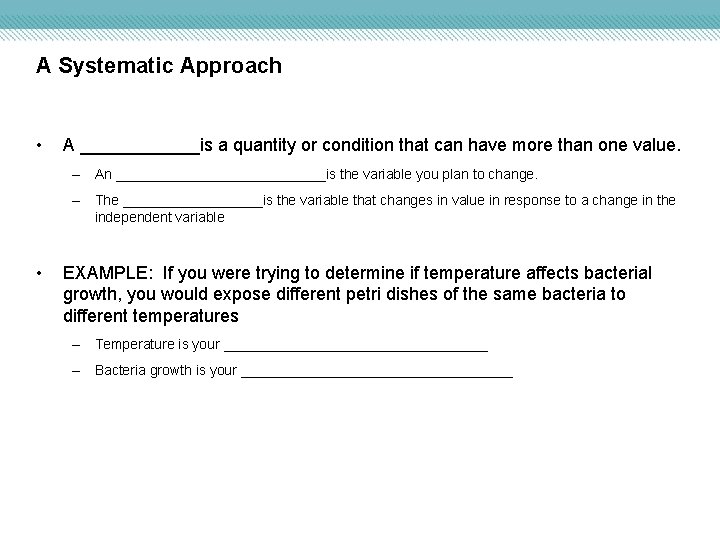
A Systematic Approach • A ______is a quantity or condition that can have more than one value. – An ______________is the variable you plan to change. – The _________is the variable that changes in value in response to a change in the independent variable • EXAMPLE: If you were trying to determine if temperature affects bacterial growth, you would expose different petri dishes of the same bacteria to different temperatures – Temperature is your _________________ – Bacteria growth is your __________________

A Systematic Approach • A ________is a standard for comparison in the experiment. • During clinical drug trials, physicians will use a double-blind study. They use 2 statistically identical groups of patients. 1 will receive the drug and 1 will receive a placebo (_____). Neither patient or physician know which group receives the drugs. • The group receiving the placebo is the control group. • A _______is a judgment based on the information obtained from the experiment. – A hypothesis is never proven, only supported or discarded. Experiments

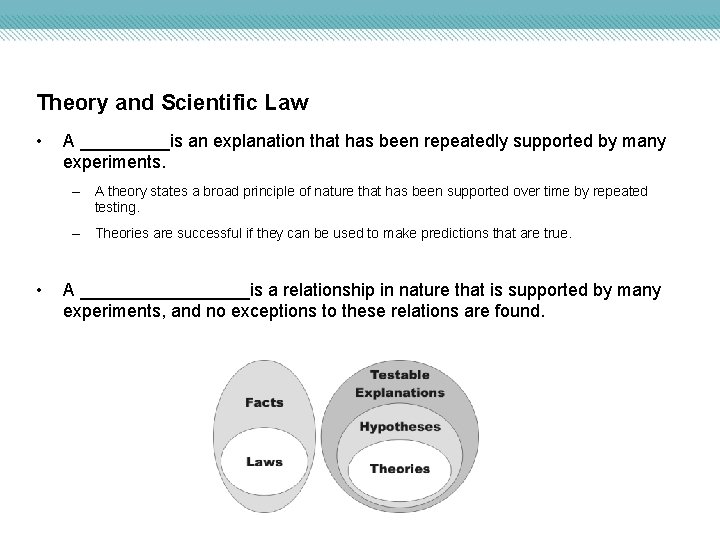
Theory and Scientific Law • A _____is an explanation that has been repeatedly supported by many experiments. – A theory states a broad principle of nature that has been supported over time by repeated testing. – Theories are successful if they can be used to make predictions that are true. • A _________is a relationship in nature that is supported by many experiments, and no exceptions to these relations are found.

Types of Scientific Investigations • ______is research to gain knowledge for the sake of knowledge itself. • ________is research undertaken to solve a specific problem. • Change discoveries occurs when scientists obtain results that are far different from what they expected. – Sometimes accidents are better! Alexander Fleming’s discovery of Penicillin has saved millions.

Review Essential Questions • What are the common steps of scientific method? • What are the similarities and differences between qualitative data and quantitative data? • In an experiment, which variable is the independent variable, which is the dependent variable, and which are controls? • What is the difference between a scientific theory and a scientific law? • How do pure research, applied research, and technology compare and contrast? Vocabulary • • Scientific method • Qualitative data • Quantitative data • Hypothesis • Experiment • Independent variable • Dependent variable • Control Conclusion Theory Scientific law Synthetic Pure research Applied research
