Introduction to Chemistry Boardworks Ltd 2003 Whats Chemistry

Introduction to Chemistry! © Boardworks Ltd 2003

What’s Chemistry? water rocks It is a branch of science which describes all the substances (materials) that make up the world around us. air earth © Boardworks Ltd 2003

Natural Materials n These materials are naturally occurring and they are not made in the laboratory. • Wool and water are examples of natural materials. © Boardworks Ltd 2003

Synthetic Materials n n n Synthetic means “artificial” or “man-made”. These materials are made in a laboratory. Examples of synthetic materials: nylon, polyester, polythene, glass and terylene. © Boardworks Ltd 2003

Chemistry has made many improvements in our lives. n Chemists manufacture items like medicines, plastics, paints, fertilisers, fuels and synthetic clothes. n n Materials which are used to manufacture various substances are commonly called CHEMICALS. n These combine with each other in chemical reactions © Boardworks Ltd 2003

States of Matter We have already learned that our world is made up of various materials. n The scientific name given to each of these materials is matter. n Matter is anything which occupies space and which has a mass (mass g/kg). n The 3 states of matter are 1. solids 2. liquids 3. gases n © Boardworks Ltd 2003

States of matter At normal temperature almost all substances exist in one of three physical states: liquids gases solids © Boardworks Ltd 2003
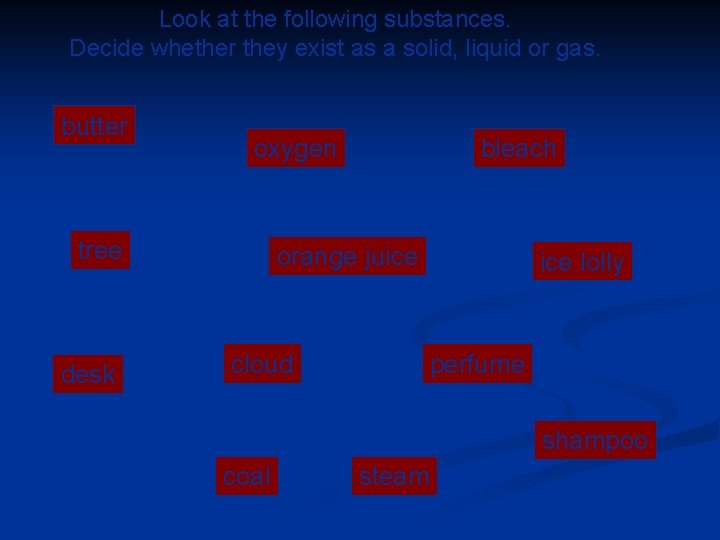
Look at the following substances. Decide whether they exist as a solid, liquid or gas. butter oxygen tree desk bleach orange juice cloud ice lolly perfume shampoo coal steam © Boardworks Ltd 2003
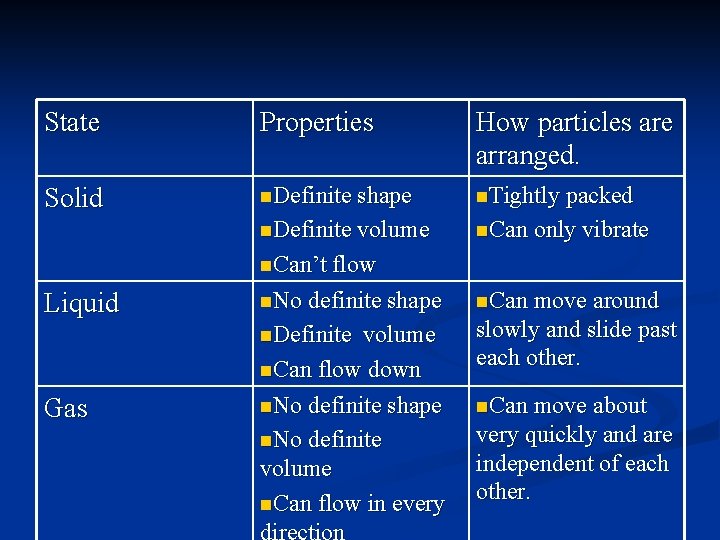
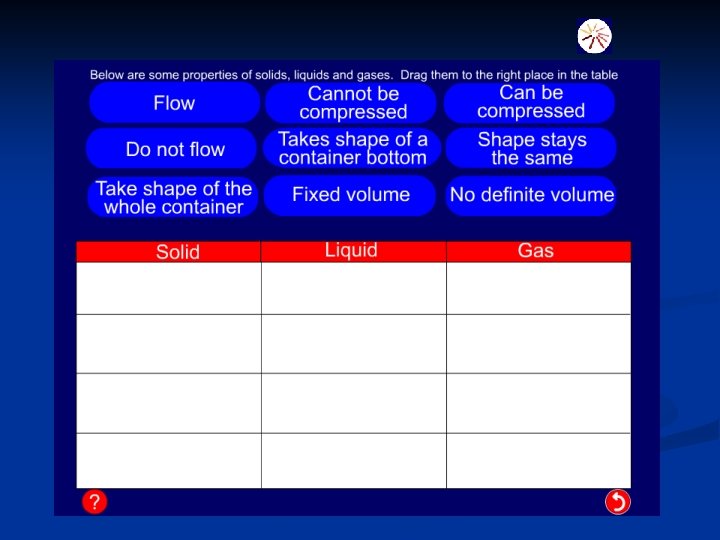
State Properties How particles are arranged. Solid n. Definite shape n. Tightly packed n. Definite volume n. Can only vibrate n. Can’t flow Liquid Gas n. No definite shape n. Can move around n. Definite slowly and slide past each other. volume n. Can flow down n. No definite shape n. No definite volume n. Can flow in every direction n. Can move about very quickly and are independent of each other. © Boardworks Ltd 2003
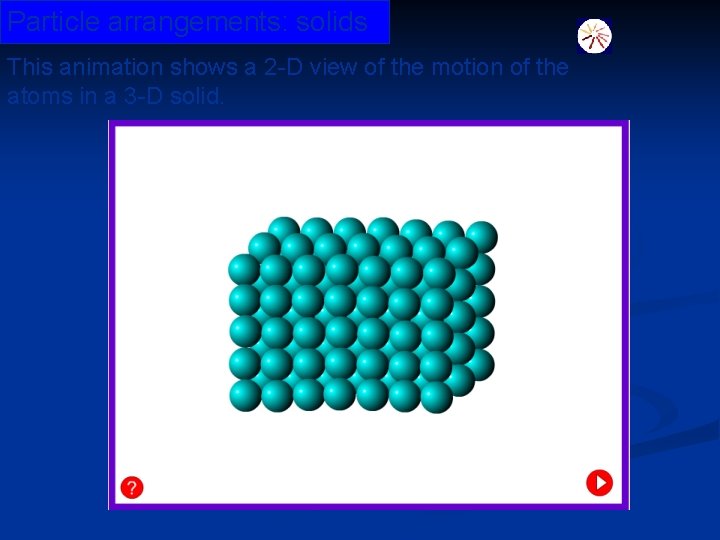
Particle arrangements: solids This animation shows a 2 -D view of the motion of the atoms in a 3 -D solid. © Boardworks Ltd 2003

Particle arrangements: liquids This animation shows a 2 -D view of the motion of the atoms in a liquid. There is no order. © Boardworks Ltd 2003
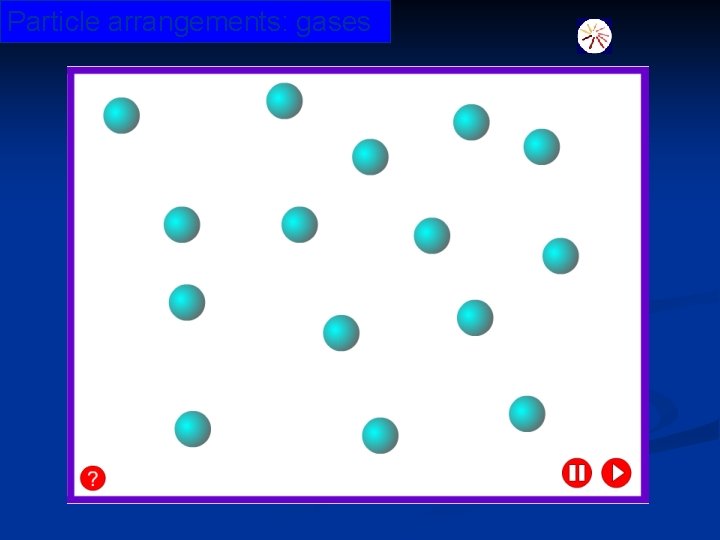
Particle arrangements: gases © Boardworks Ltd 2003
© Boardworks Ltd 2003
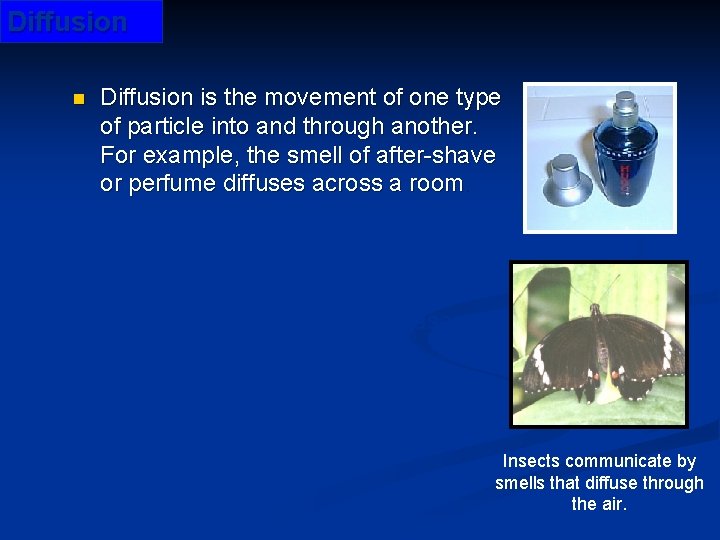
Diffusion n Diffusion is the movement of one type of particle into and through another. For example, the smell of after-shave or perfume diffuses across a room. Can you explain these facts? • Diffusion occurs both in liquids and gases but hardly at all in solids. • It happens more quickly for gases than for liquids. • It happens more quickly at warm temperatures than at cooler temperatures. Insects communicate by smells that diffuse through the air. © Boardworks Ltd 2003

Diffusion © Boardworks Ltd 2003
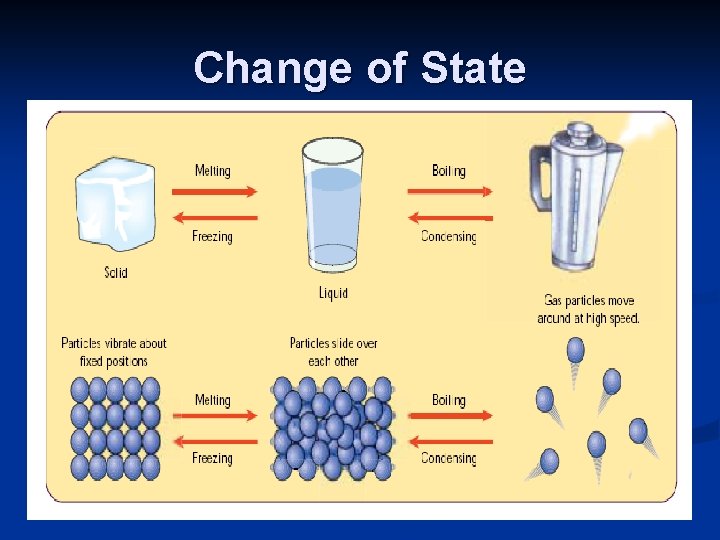
Change of State © Boardworks Ltd 2003
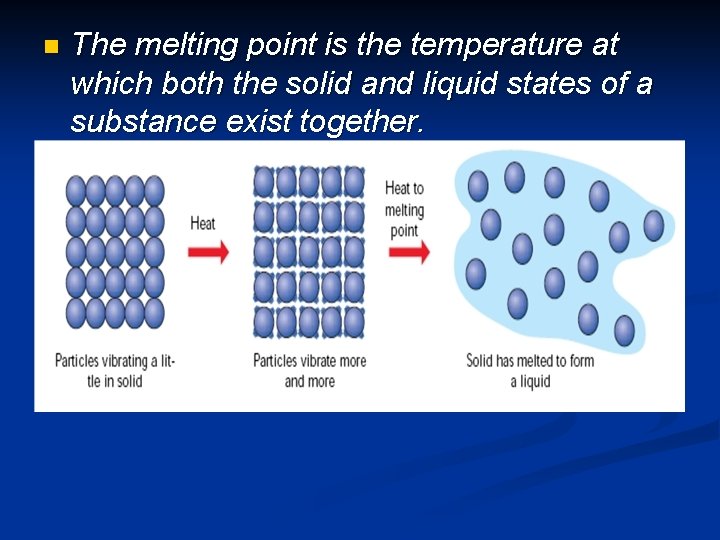
n The melting point is the temperature at which both the solid and liquid states of a substance exist together. © Boardworks Ltd 2003
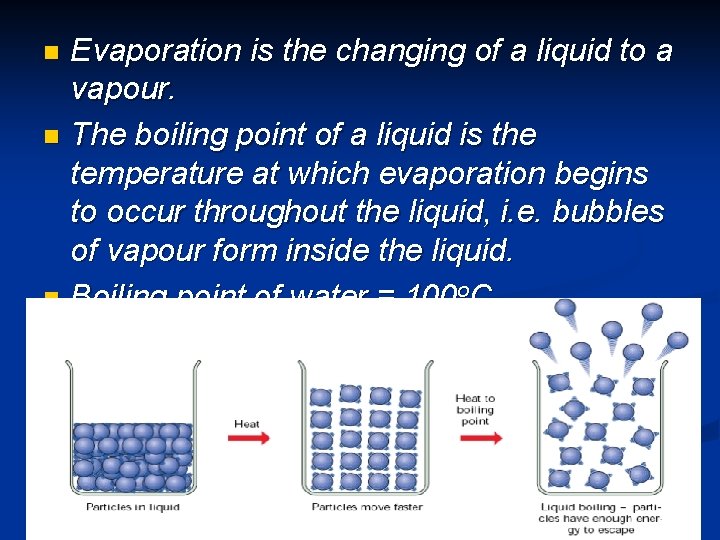
Evaporation is the changing of a liquid to a vapour. n The boiling point of a liquid is the temperature at which evaporation begins to occur throughout the liquid, i. e. bubbles of vapour form inside the liquid. n Boiling point of water = 100 o. C n Boiling point of alcohol = 78 o. C n © Boardworks Ltd 2003
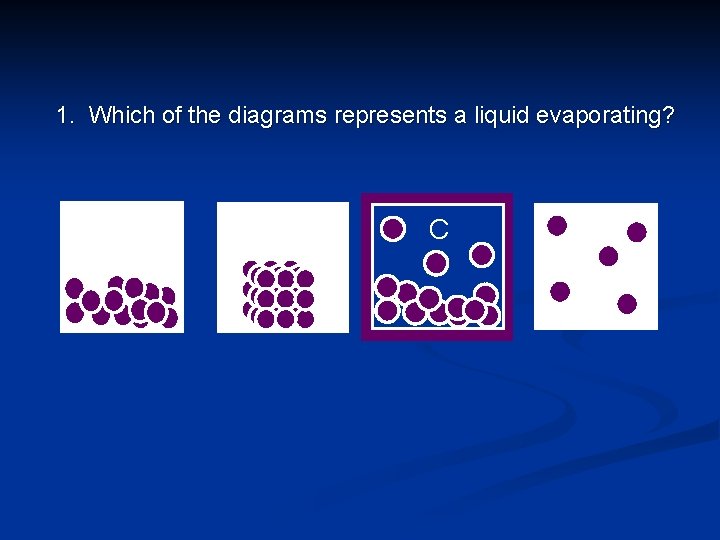
1. Which of the diagrams represents a liquid evaporating? A B C D © Boardworks Ltd 2003

2. Which of the following describes a liquid? A B C D compressible, fluid non-compressible, fluid compressible, no fixed shape non-compressible, very low density © Boardworks Ltd 2003

3. Which of these is only true for a gas? A can flow B exerts pressure on its container C will occupy the whole of the container it is placed in D has no shape of its own © Boardworks Ltd 2003
- Slides: 21