Introduction to Chemical Reactions What is a Chemical






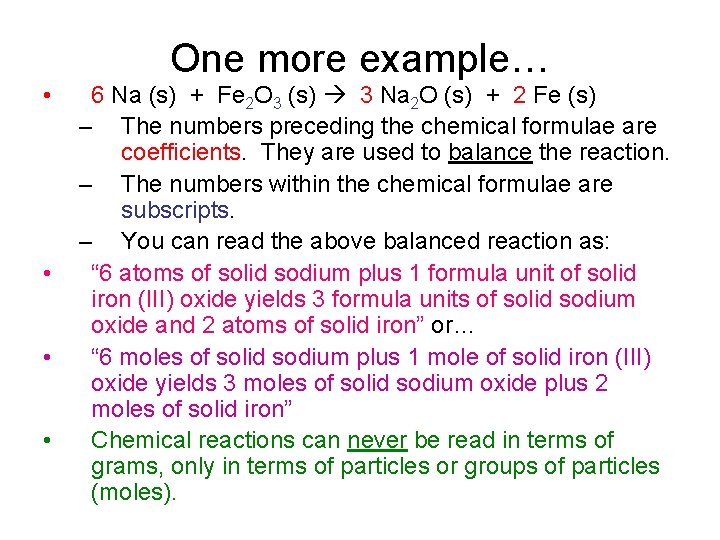











- Slides: 74

Introduction to Chemical Reactions

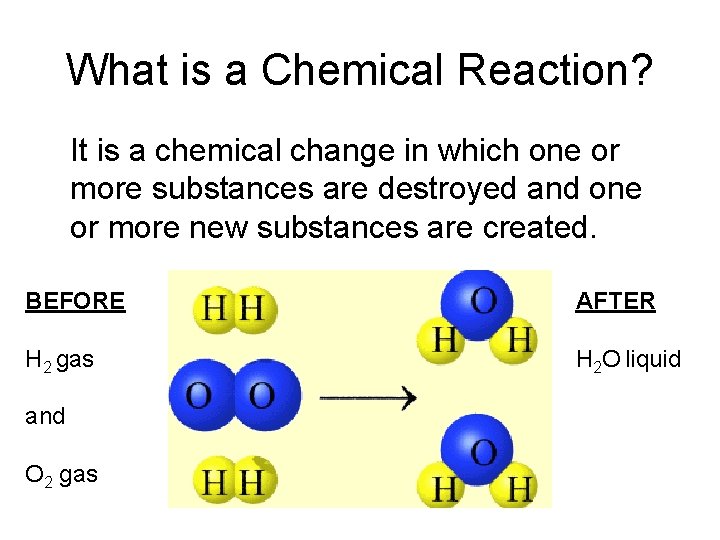
What is a Chemical Reaction? It is a chemical change in which one or more substances are destroyed and one or more new substances are created. BEFORE AFTER H 2 gas H 2 O liquid and O 2 gas

Parts of a Chemical Reaction Reactants Products Reactants: Substances that are destroyed by the chemical change (bonds break). break Products: Substances created by the chemical change (new bonds form). form The arrow ( ) is read as “yields”.

Other symbols in chemical reactions • • (s) = solid (l) = liquid (g) = gas (aq) = aqueous solution (the substance is dissolved in H 2 O) • “+” separates two or more reactants or products • “ ” yield sign separates reactants from products

Evidence for a Chemical Reaction 1) Evolution of light or heat.

Evidence for a Chemical Reaction 2) Temperature change (increase or decrease) to the surroundings.

Evidence for a Chemical Reaction 3) Formation of a gas (bubbling or an odor) other than boiling.

Evidence for a Chemical Reaction 4) Color change (due to the formation of a new substance).

Evidence for a Chemical Reaction 5) Formation of a precipitate (a new solid forms) from the reaction of two aqueous solutions.

Word Equations • Statements that indicate the reactants and products in a chemical reaction. • Ex. Iron (s) + chlorine (g) iron (III) chloride (s) • This is read as: “Solid iron and chlorine gas react (combine) to produce solid iron (III) chloride”

Translating Word Equations to Skeleton Equations • A skeleton equation uses chemical formulas rather than words to identify the reactants and products of a chemical reaction. • The word equation Iron (s) + chlorine (g) iron (III) chloride (s) • The skeleton equation Fe(s) + Cl 2(g) Fe. Cl 3 (s) A skeleton equation is not yet “balanced” by coefficients!
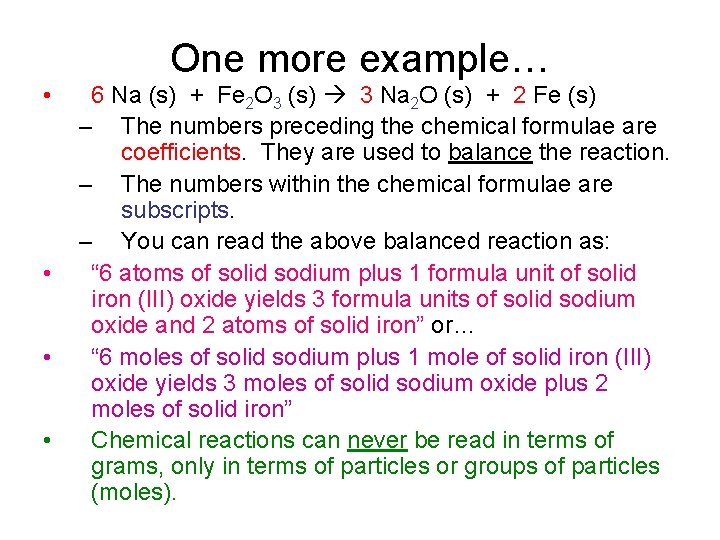
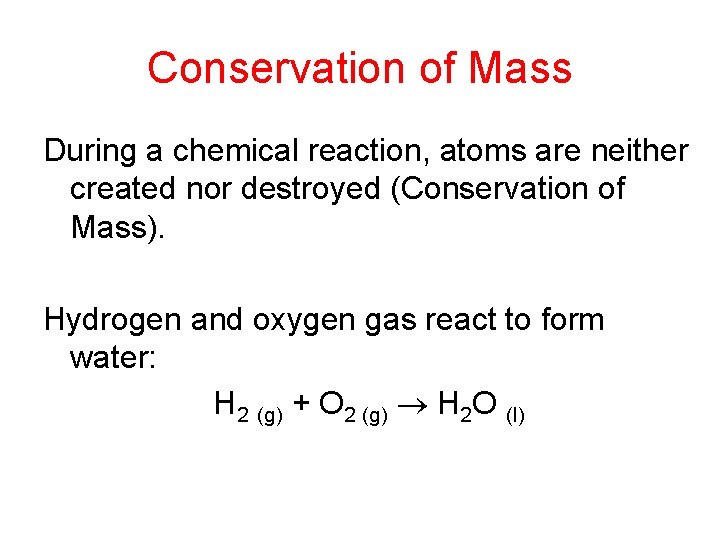
One more example… • • 6 Na (s) + Fe 2 O 3 (s) 3 Na 2 O (s) + 2 Fe (s) – The numbers preceding the chemical formulae are coefficients. They are used to balance the reaction. – The numbers within the chemical formulae are subscripts. – You can read the above balanced reaction as: “ 6 atoms of solid sodium plus 1 formula unit of solid iron (III) oxide yields 3 formula units of solid sodium oxide and 2 atoms of solid iron” or… “ 6 moles of solid sodium plus 1 mole of solid iron (III) oxide yields 3 moles of solid sodium oxide plus 2 moles of solid iron” Chemical reactions can never be read in terms of grams, only in terms of particles or groups of particles (moles).

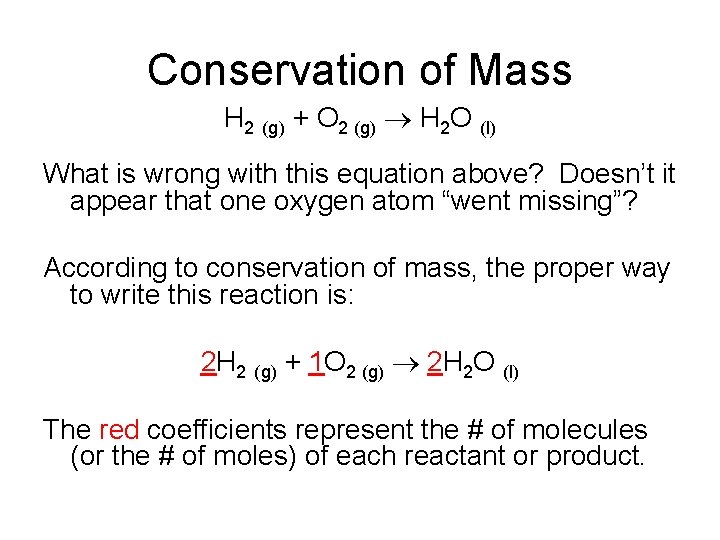
Conservation of Mass During a chemical reaction, atoms are neither created nor destroyed (Conservation of Mass). Hydrogen and oxygen gas react to form water: H 2 (g) + O 2 (g) H 2 O (l)

Conservation of Mass H 2 (g) + O 2 (g) H 2 O (l) What is wrong with this equation above? Doesn’t it appear that one oxygen atom “went missing”? According to conservation of mass, the proper way to write this reaction is: 2 H 2 (g) + 1 O 2 (g) 2 H 2 O (l) The red coefficients represent the # of molecules (or the # of moles) of each reactant or product.

Not All Properties are Conserved During Chemical Reactions! CONSERVED NOT CONSERVED Mass Types of atoms Number of each atom Color Physical state (solid, liquid, gas) Volume Number of moles of reactants/products

TYPES OF CHEMICAL REACTIONS

There are 5 basic types…. • Single Replacement (Displacement) (Redox) • Double Replacement (Displacement) (Metathesis) • Synthesis (Combination) • Decomposition • Combustion

1) SINGLE REPLACEMENT REACTION A single uncombined element replaces another element in an ionic compound. There are two reactants and two products. Ex: Zn + Cu. SO 4 Zn. SO 4 + Cu

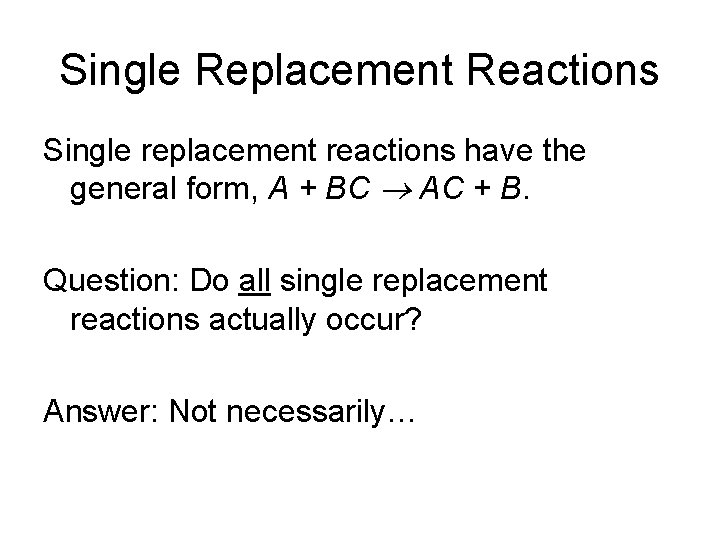
Single Replacement Reactions Single replacement reactions have the general form, A + BC AC + B. Question: Do all single replacement reactions actually occur? Answer: Not necessarily…

Single Replacement Reactions Examine the reaction: Zn + Cu. SO 4 Zn. SO 4 + Cu This reaction does occur!’ Now let’s try: Cu + Zn. SO 4 No Reaction Conclusion: Zn will replace Cu in solution, but not vice versa!

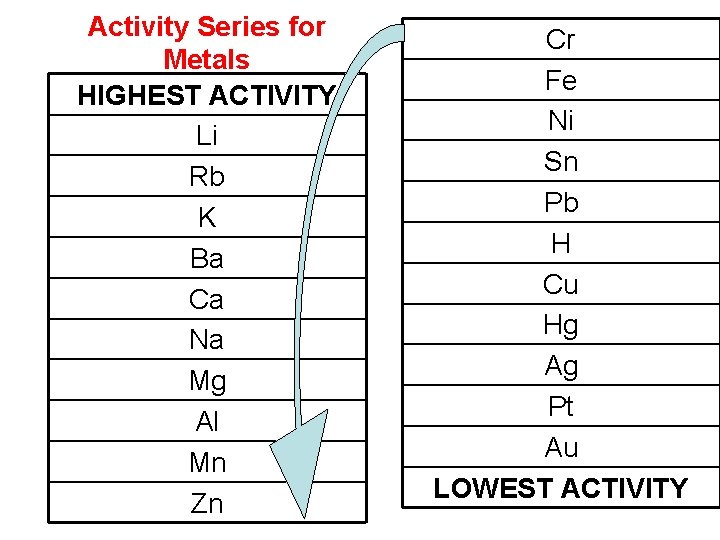
Single Replacement Reactions How do we know which reactions will occur and which ones will not? We look at the “activity series”. Elements with higher activities replace elements with lower activities during a single-replacement reaction, but not viceversa.

Activity Series for Metals HIGHEST ACTIVITY Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H Cu Hg Ag Pt Au LOWEST ACTIVITY

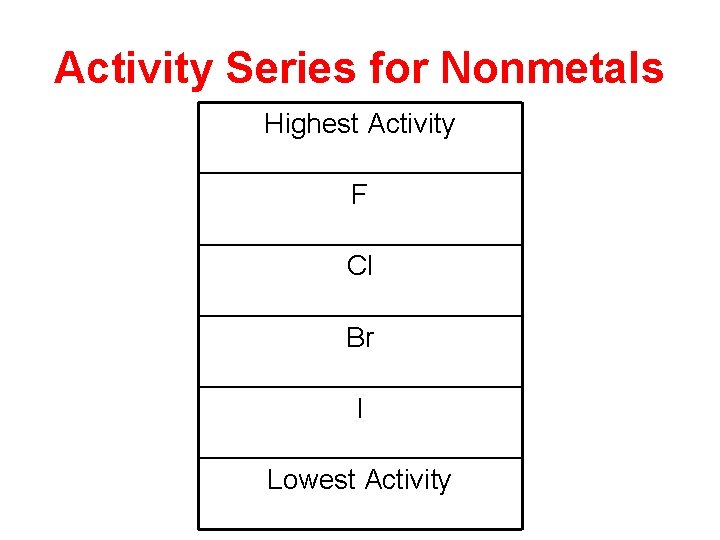
Activity Series for Nonmetals Highest Activity F Cl Br I Lowest Activity

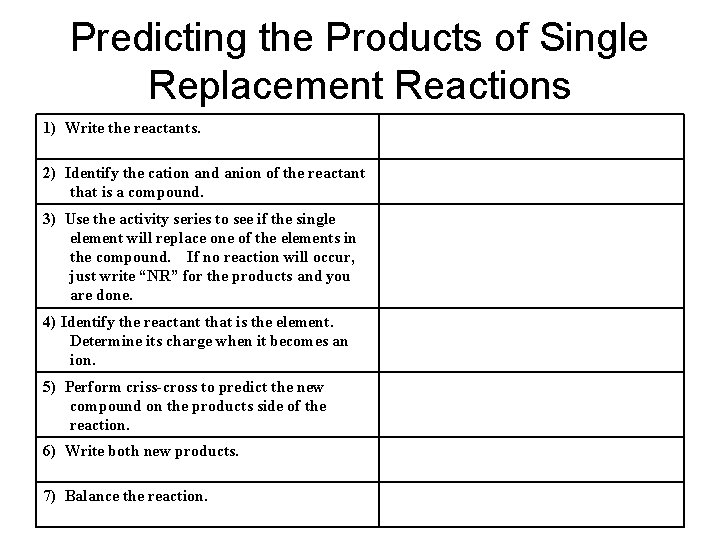
Predicting the Products of Single Replacement Reactions 1) Write the reactants. 2) Identify the cation and anion of the reactant that is a compound. 3) Use the activity series to see if the single element will replace one of the elements in the compound. If no reaction will occur, just write “NR” for the products and you are done. 4) Identify the reactant that is the element. Determine its charge when it becomes an ion. 5) Perform criss-cross to predict the new compound on the products side of the reaction. 6) Write both new products. 7) Balance the reaction.

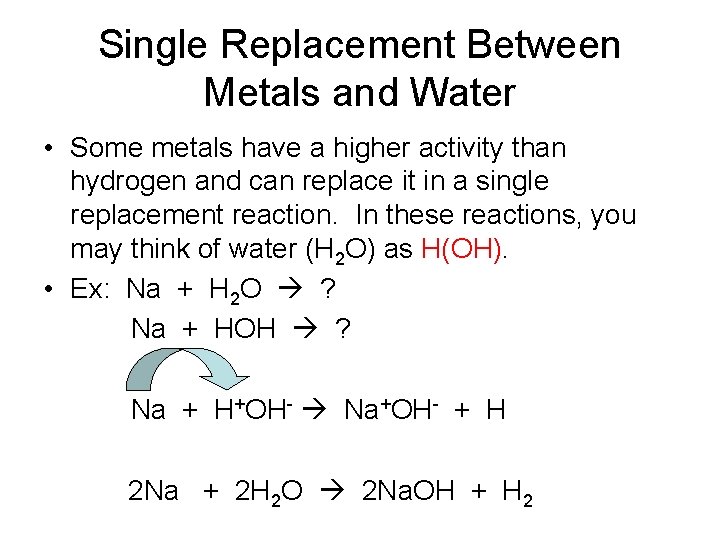
Single Replacement Between Metals and Water • Some metals have a higher activity than hydrogen and can replace it in a single replacement reaction. In these reactions, you may think of water (H 2 O) as H(OH). • Ex: Na + H 2 O ? Na + HOH ? Na + H+OH- Na+OH- + H 2 Na + 2 H 2 O 2 Na. OH + H 2

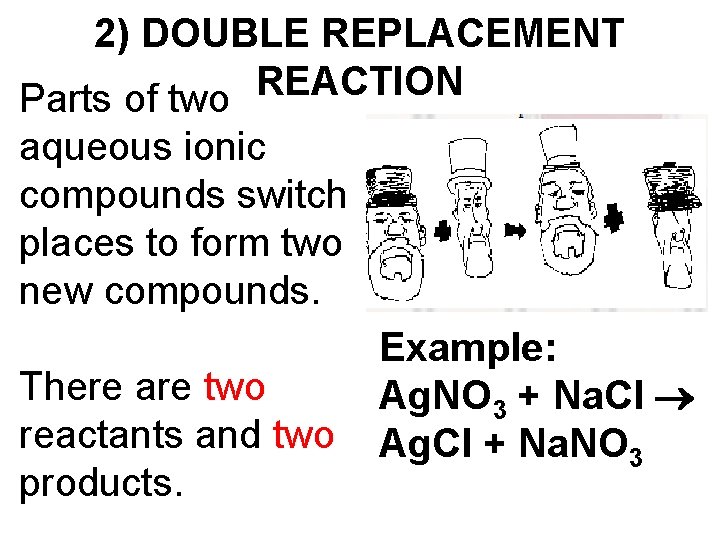
2) DOUBLE REPLACEMENT REACTION Parts of two aqueous ionic compounds switch places to form two new compounds. There are two reactants and two products. Example: Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3

Double Replacement Reactions The general form of a double replacement reaction is: AB + CD AD + CB Just like single replacement reactions, not all double replacement reactions actually occur. We can experimentally attempt a D. R. reaction. The reaction occurs if: 1) 2) 3) A solid precipitate is produced, or A gas is produced, or Water is produced. If none of the above are produced and both products are (aq), then there is no reaction (NR)!

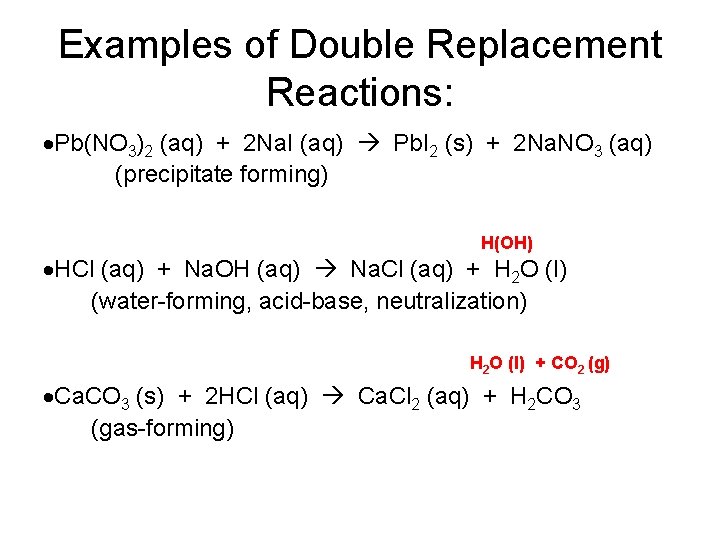
Examples of Double Replacement Reactions: Pb(NO 3)2 (aq) + 2 Na. I (aq) Pb. I 2 (s) + 2 Na. NO 3 (aq) (precipitate forming) H(OH) HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l) (water-forming, acid-base, neutralization) H 2 O (l) + CO 2 (g) Ca. CO 3 (s) + 2 HCl (aq) Ca. Cl 2 (aq) + H 2 CO 3 (gas-forming)

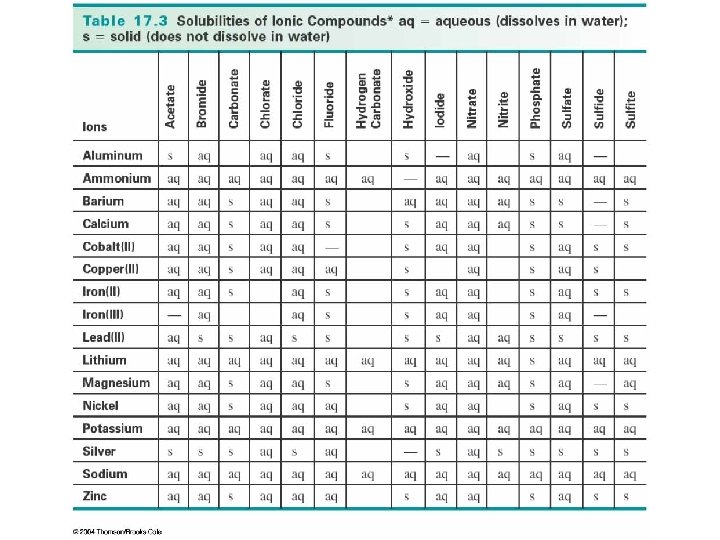
How do you determine if one of the products of a double replacement reaction will be a precipitate? • Use the solubility rules…. Soluble compounds These compounds break down when put in water. Example: In water, Na. Cl Na 1+ and Cl 1 -. We say that Na. Cl… has dissolved. is soluble. forms an aqueous solution (aq).

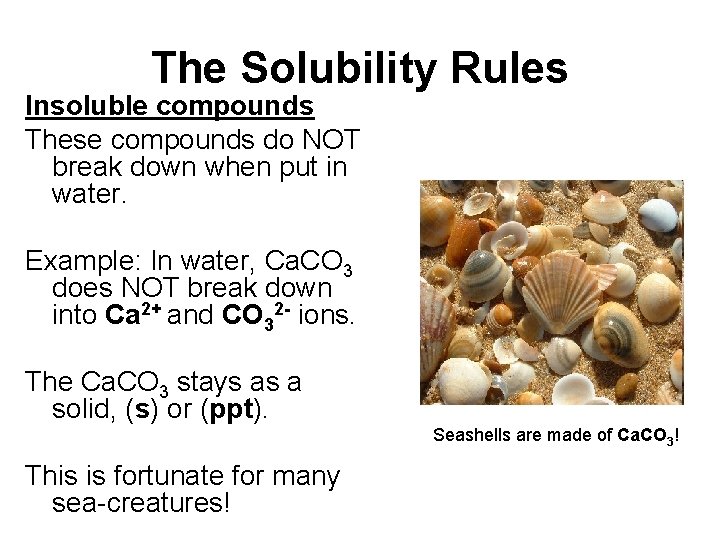
The Solubility Rules Insoluble compounds These compounds do NOT break down when put in water. Example: In water, Ca. CO 3 does NOT break down into Ca 2+ and CO 32 - ions. The Ca. CO 3 stays as a solid, (s) or (ppt). Seashells are made of Ca. CO 3! This is fortunate for many sea-creatures!

The Solubility Rules You do not have to memorize these rules, but you do have to know how to use them to determine if a product is a precipitate. See the chart on the next slide…. . Let’s check Na. Cl and Ca. CO 3… Are these compounds soluble or insoluble in aqueous solution?

Solubility Rules Chart

Predicting the Products of Double Replacement Reactions… Step 1) Write the two reactants (both are ionic compounds) 2) Identify the cations and anions in both of the compound reactants 3) Pair up each cation with the anion from the other compound (i. e. – switch the cations) 4) Write the formula for each product using the criss-cross method 5) Write the complete equation for the double replacement reaction 6) Balance the equation. 7) Use the solubility rules chart to figure out which product is a precipitate (s) and which product is an aqueous solution (aq). If both products are (aq) it is really not a reaction. Example

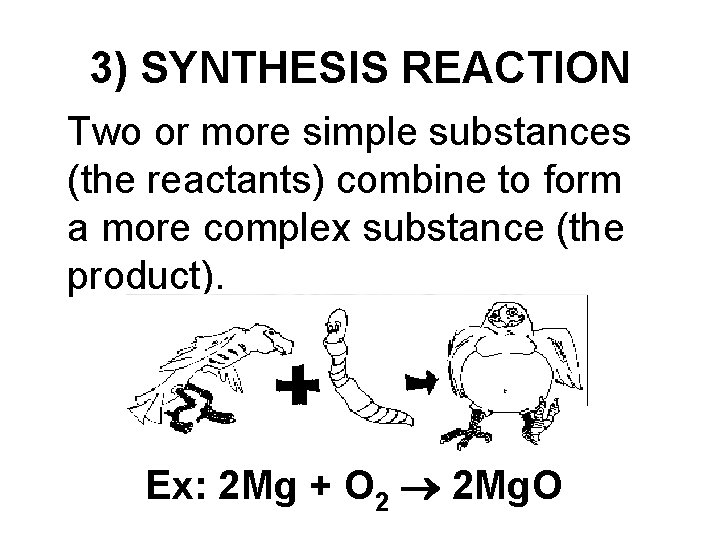
3) SYNTHESIS REACTION Two or more simple substances (the reactants) combine to form a more complex substance (the product). Ex: 2 Mg + O 2 2 Mg. O

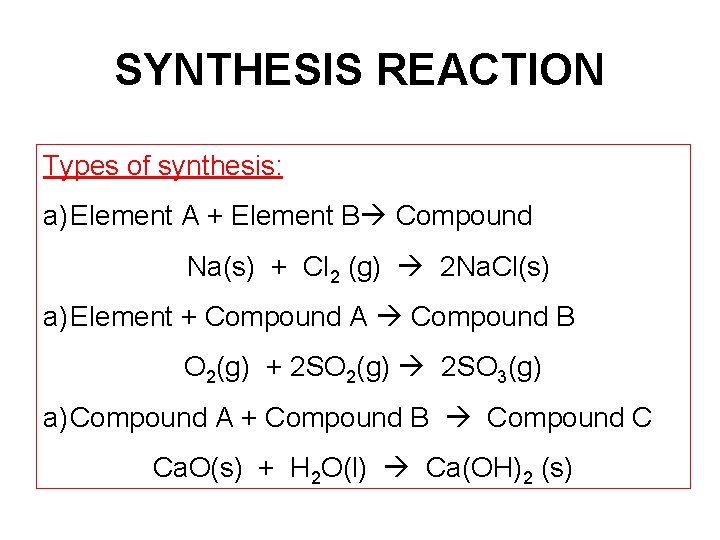
SYNTHESIS REACTION Types of synthesis: a) Element A + Element B Compound Na(s) + Cl 2 (g) 2 Na. Cl(s) a) Element + Compound A Compound B O 2(g) + 2 SO 2(g) 2 SO 3(g) a) Compound A + Compound B Compound C Ca. O(s) + H 2 O(l) Ca(OH)2 (s)

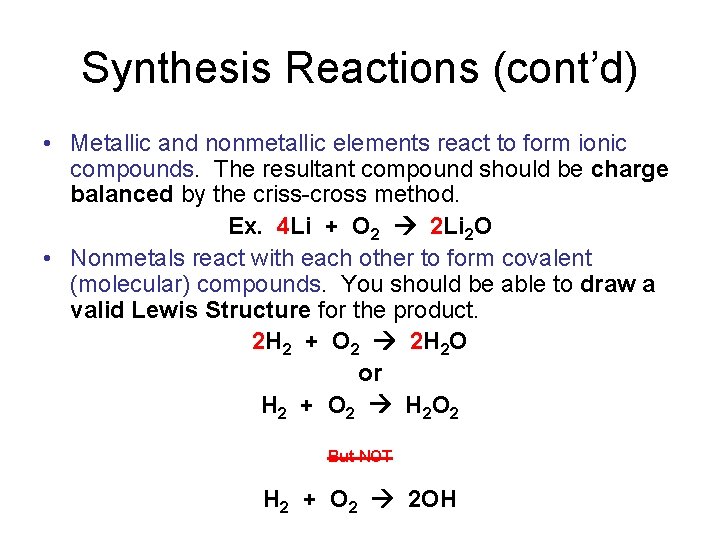
Synthesis Reactions (cont’d) • Metallic and nonmetallic elements react to form ionic compounds. The resultant compound should be charge balanced by the criss-cross method. Ex. 4 Li + O 2 2 Li 2 O • Nonmetals react with each other to form covalent (molecular) compounds. You should be able to draw a valid Lewis Structure for the product. 2 H 2 + O 2 2 H 2 O or H 2 + O 2 H 2 O 2 But NOT H 2 + O 2 2 OH

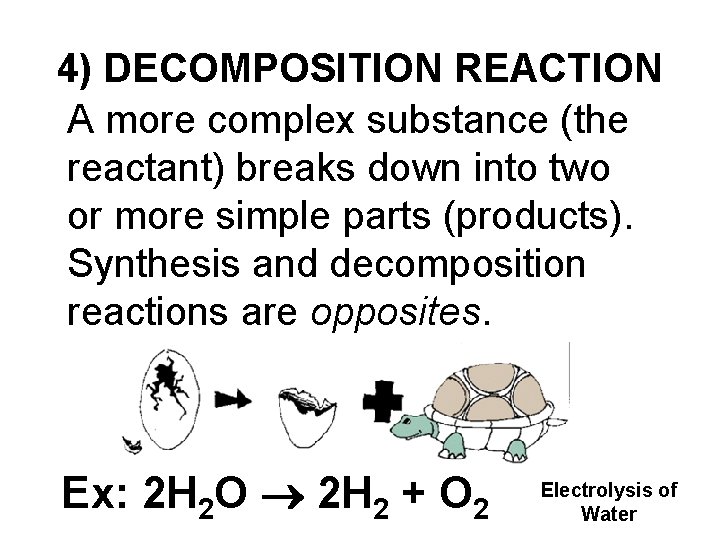
4) DECOMPOSITION REACTION A more complex substance (the reactant) breaks down into two or more simple parts (products). Synthesis and decomposition reactions are opposites. Ex: 2 H 2 O 2 H 2 + O 2 Electrolysis of Water

DECOMPOSITION REACTIONS (Cont’d) Decomposition of a compound produces two or more elements and/or compounds The products are always simpler than the reactant. Gases are often produced (H 2, N 2, O 2, CO 2, etc. ) in the decomposition of covalent compounds. Ionic compounds may be decomposed into pure elements by using electricity (electrolysis). This is how pure metals are obtained from salts.

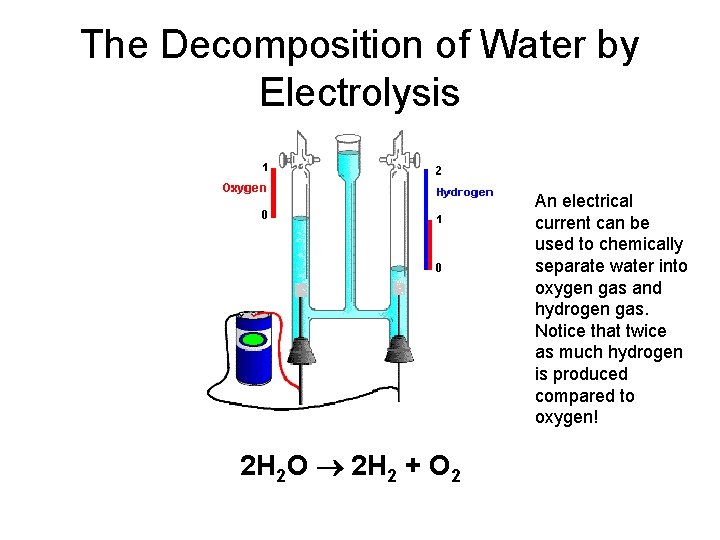
The Decomposition of Water by Electrolysis An electrical current can be used to chemically separate water into oxygen gas and hydrogen gas. Notice that twice as much hydrogen is produced compared to oxygen! 2 H 2 O 2 H 2 + O 2

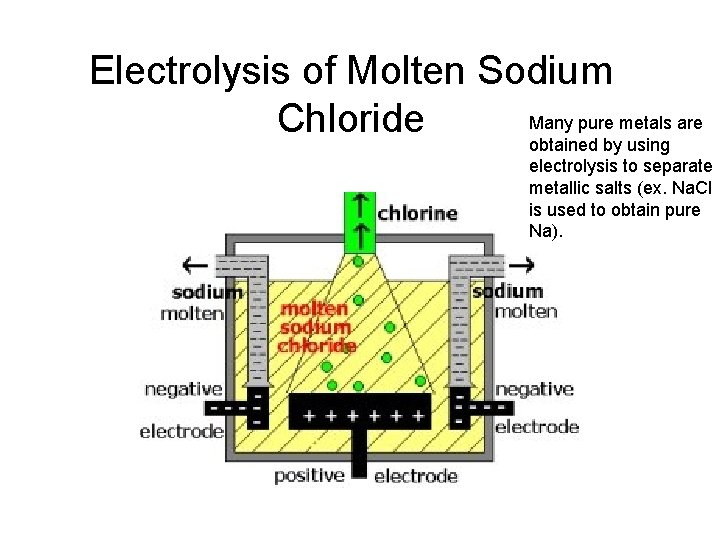
Electrolysis of Molten Sodium Many pure metals are Chloride obtained by using electrolysis to separate metallic salts (ex. Na. Cl is used to obtain pure Na).

5) COMBUSTION REACTIONS a) All involve oxygen (O 2) as a reactant, combining with another substance b) All combustion reactions are exothermic c) Complete combustion of a hydrocarbon always produces CO 2 and H 2 O d) Incomplete combustion of a hydrocarbon will produce CO and possibly C (black carbon soot) as well Ex: CH 4 + 2 O 2 => CO 2 + 2 H 2 O Ex: CH 4 + 1. 5 O 2 => CO + 2 H 2 O Ex: CH 4 + O 2 => C + 2 H 2 O (complete combustion – blue flame) (incomplete combustion – yellow flame, soot)

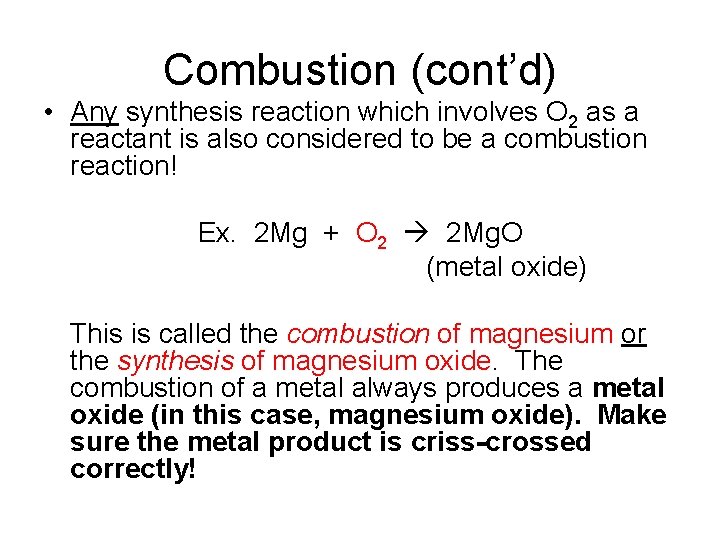
Combustion (cont’d) • Any synthesis reaction which involves O 2 as a reactant is also considered to be a combustion reaction! Ex. 2 Mg + O 2 2 Mg. O (metal oxide) This is called the combustion of magnesium or the synthesis of magnesium oxide. The combustion of a metal always produces a metal oxide (in this case, magnesium oxide). Make sure the metal product is criss-crossed correctly!

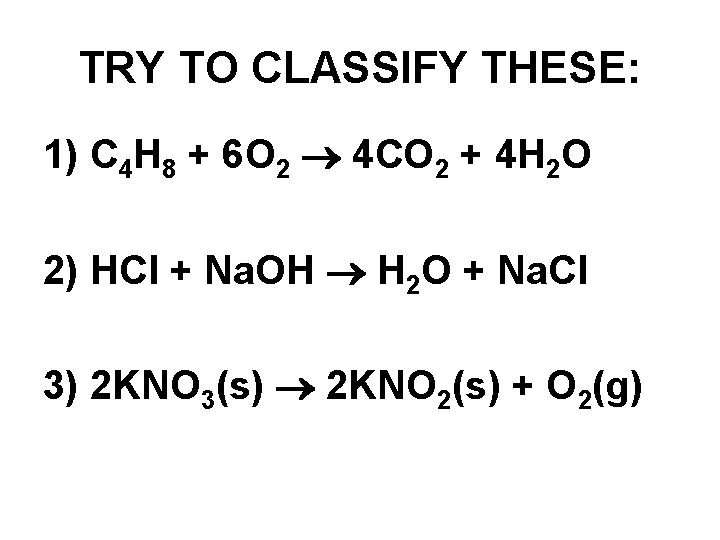
TRY TO CLASSIFY THESE: 1) C 4 H 8 + 6 O 2 4 CO 2 + 4 H 2 O 2) HCl + Na. OH H 2 O + Na. Cl 3) 2 KNO 3(s) 2 KNO 2(s) + O 2(g)

TRY TO CLASSIFY THESE: 4) 2 Ag + S Ag 2 S 5) Mg. CO 3(s) Mg. O(s) + CO 2(g) 6) Cl 2 + 2 KBr 2 KCl + Br 2

Check Your Answers… 1) 2) 3) 4) 5) 6) Combustion (of a hydrocarbon) Double replacement (water forming) Decomposition Synthesis Decomposition Single Replacement

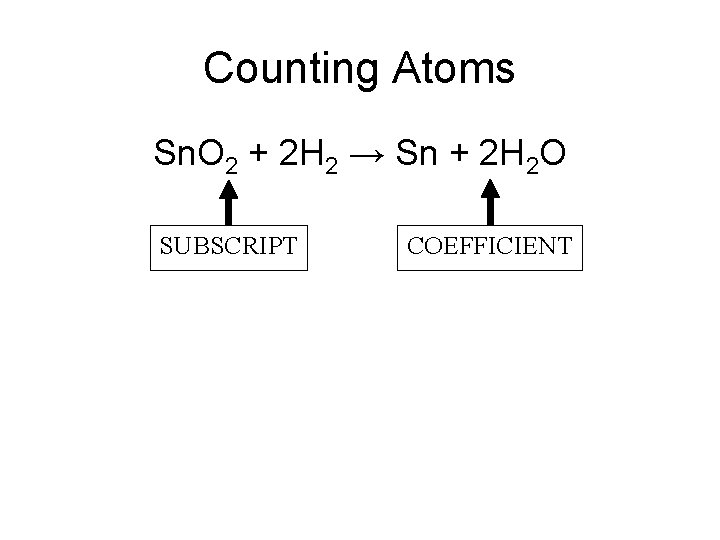
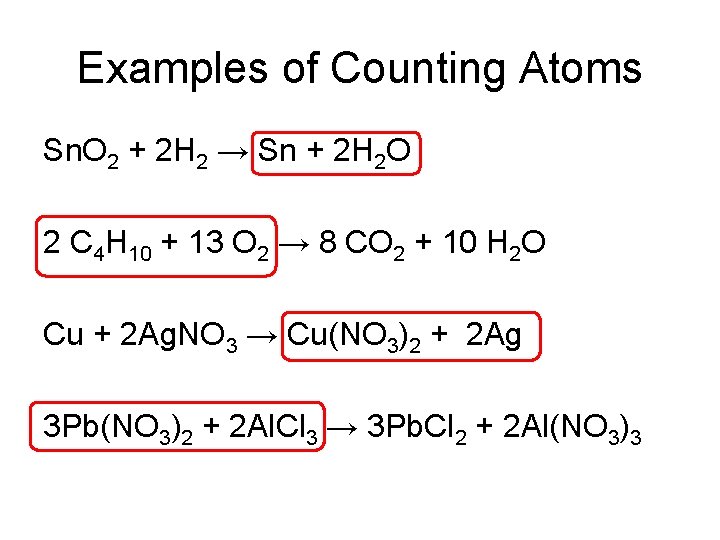
Counting Atoms Sn. O 2 + 2 H 2 → Sn + 2 H 2 O SUBSCRIPT COEFFICIENT

Rules for Counting Atoms 1) Coefficients propagate to the right through the entire compound, whether or not parentheses are present. 2) Subscripts affect only the element to the left of the subscript, unless… 3) If a subscript occurs to the right of a parentheses, the subscript propagates to the left through the parentheses. 4) When a coefficient and subscript “meet”, you must multiply the two.

Examples of Counting Atoms Sn. O 2 + 2 H 2 → Sn + 2 H 2 O 2 C 4 H 10 + 13 O 2 → 8 CO 2 + 10 H 2 O Cu + 2 Ag. NO 3 → Cu(NO 3)2 + 2 Ag 3 Pb(NO 3)2 + 2 Al. Cl 3 → 3 Pb. Cl 2 + 2 Al(NO 3)3

Classwork Complete “The Count” worksheet on counting atoms in chemical reactions.

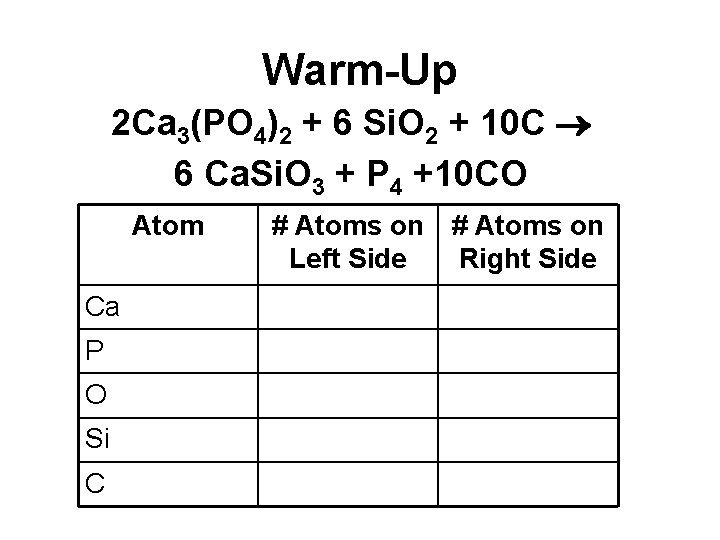
Warm-Up 2 Ca 3(PO 4)2 + 6 Si. O 2 + 10 C 6 Ca. Si. O 3 + P 4 +10 CO Atom Ca P O Si C # Atoms on Left Side Right Side

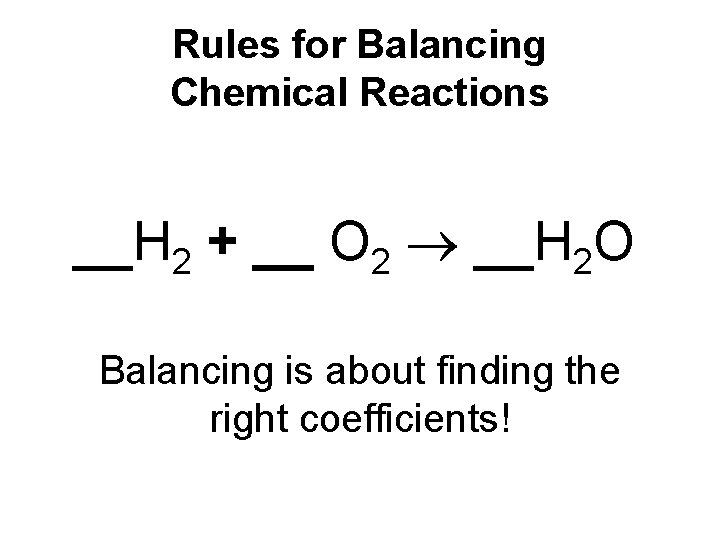
Rules for Balancing Chemical Reactions __H 2 + __ O 2 __H 2 O Balancing is about finding the right coefficients!

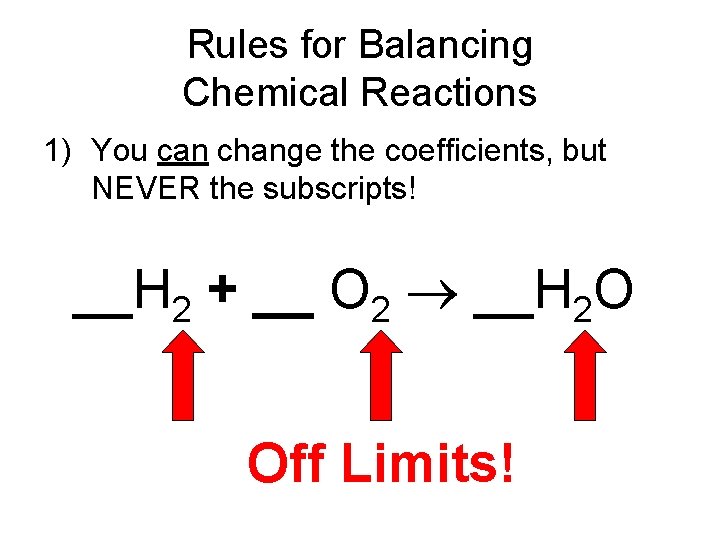
Rules for Balancing Chemical Reactions 1) You can change the coefficients, but NEVER the subscripts! __H 2 + __ O 2 __H 2 O Off Limits!

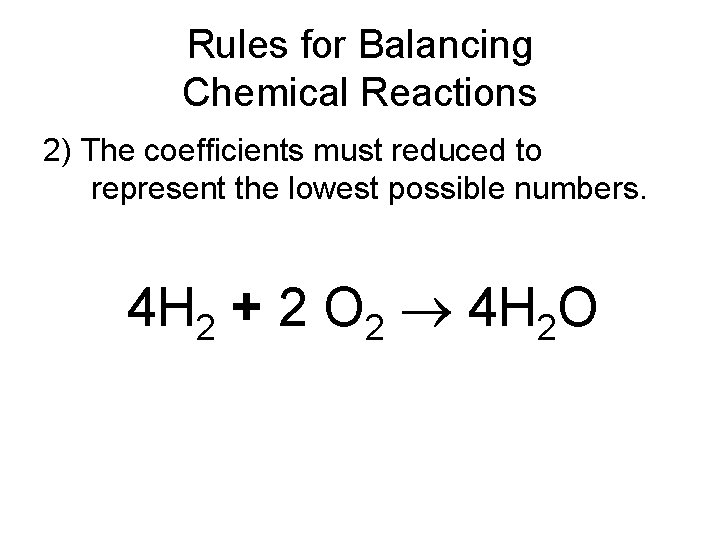
Rules for Balancing Chemical Reactions 2) The coefficients must reduced to represent the lowest possible numbers. 4 H 2 + 2 O 2 4 H 2 O

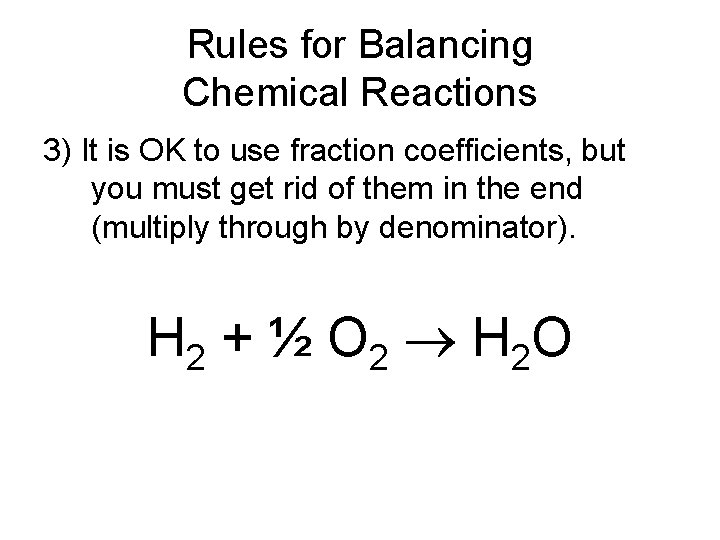
Rules for Balancing Chemical Reactions 3) It is OK to use fraction coefficients, but you must get rid of them in the end (multiply through by denominator). H 2 + ½ O 2 H 2 O

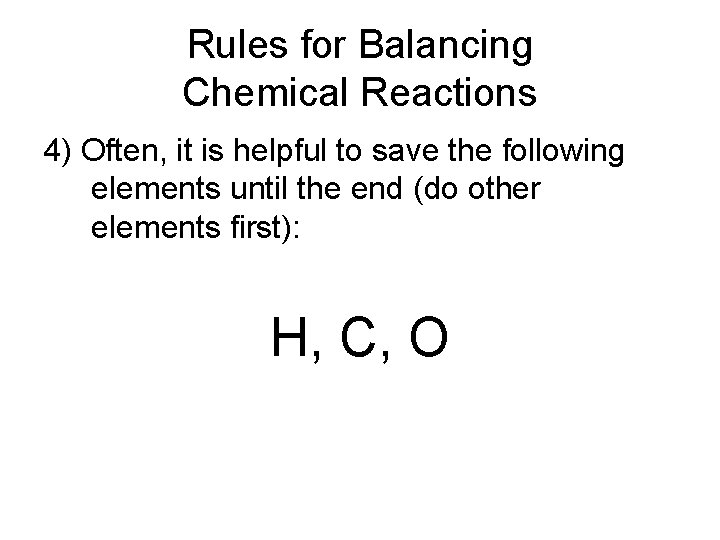
Rules for Balancing Chemical Reactions 4) Often, it is helpful to save the following elements until the end (do other elements first): H, C, O

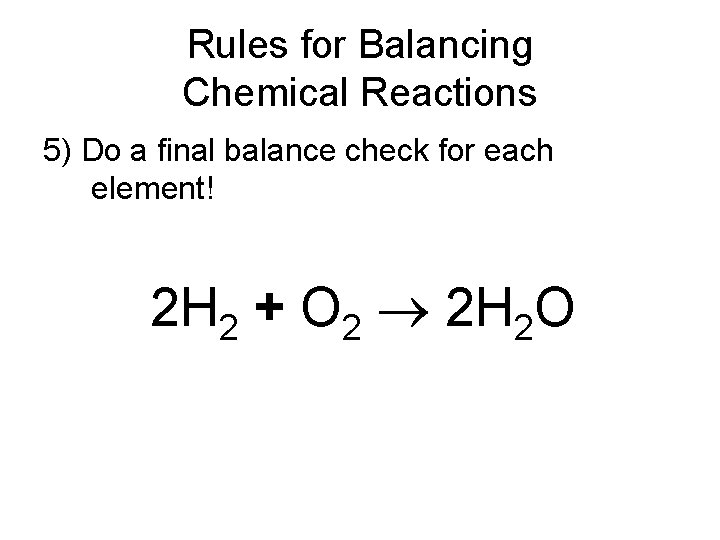
Rules for Balancing Chemical Reactions 5) Do a final balance check for each element! 2 H 2 + O 2 2 H 2 O

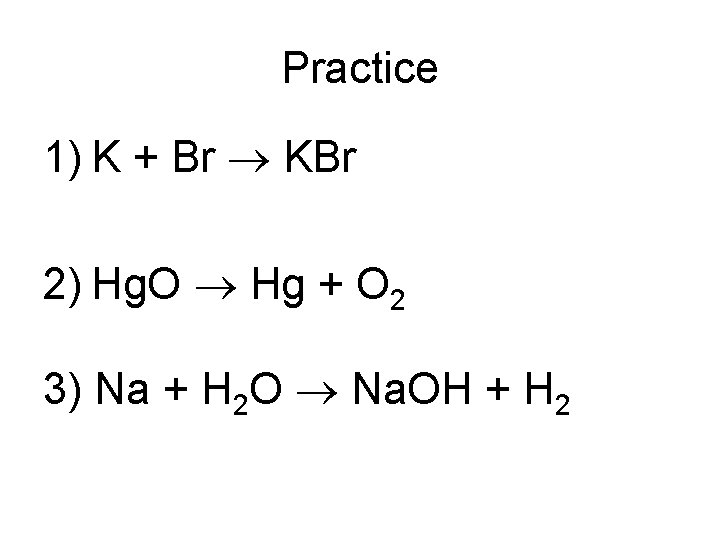
Practice 1) K + Br KBr 2) Hg. O Hg + O 2 3) Na + H 2 O Na. OH + H 2

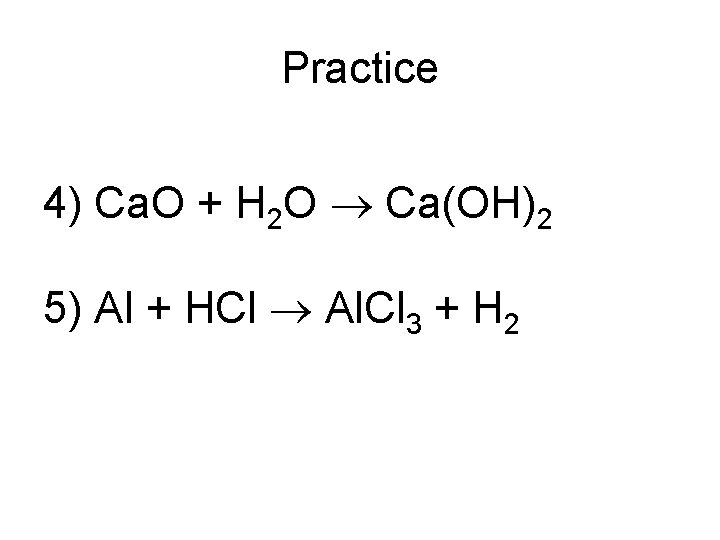
Practice 4) Ca. O + H 2 O Ca(OH)2 5) Al + HCl Al. Cl 3 + H 2

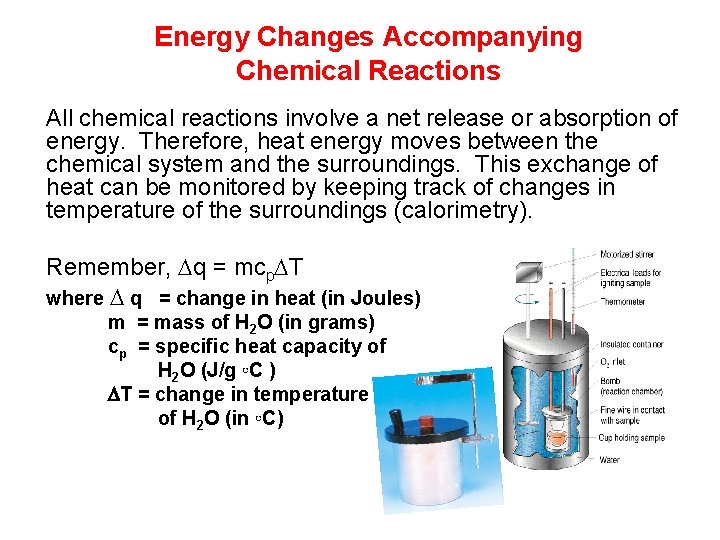
Energy Changes Accompanying Chemical Reactions All chemical reactions involve a net release or absorption of energy. Therefore, heat energy moves between the chemical system and the surroundings. This exchange of heat can be monitored by keeping track of changes in temperature of the surroundings (calorimetry). Remember, q = mcp T where q = change in heat (in Joules) m = mass of H 2 O (in grams) cp = specific heat capacity of H 2 O (J/g ◦C ) T = change in temperature of H 2 O (in ◦C)

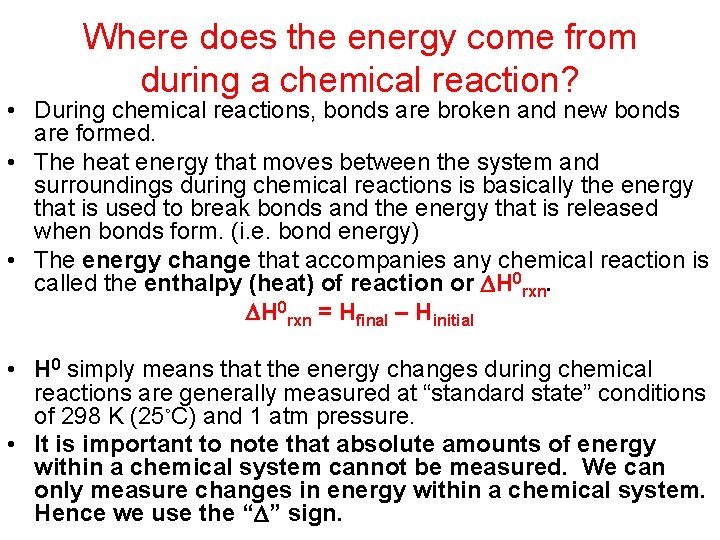
Where does the energy come from during a chemical reaction? • During chemical reactions, bonds are broken and new bonds are formed. • The heat energy that moves between the system and surroundings during chemical reactions is basically the energy that is used to break bonds and the energy that is released when bonds form. (i. e. bond energy) • The energy change that accompanies any chemical reaction is called the enthalpy (heat) of reaction or H 0 rxn = Hfinal – Hinitial • H 0 simply means that the energy changes during chemical reactions are generally measured at “standard state” conditions of 298 K (25◦C) and 1 atm pressure. • It is important to note that absolute amounts of energy within a chemical system cannot be measured. We can only measure changes in energy within a chemical system. Hence we use the “ ” sign.

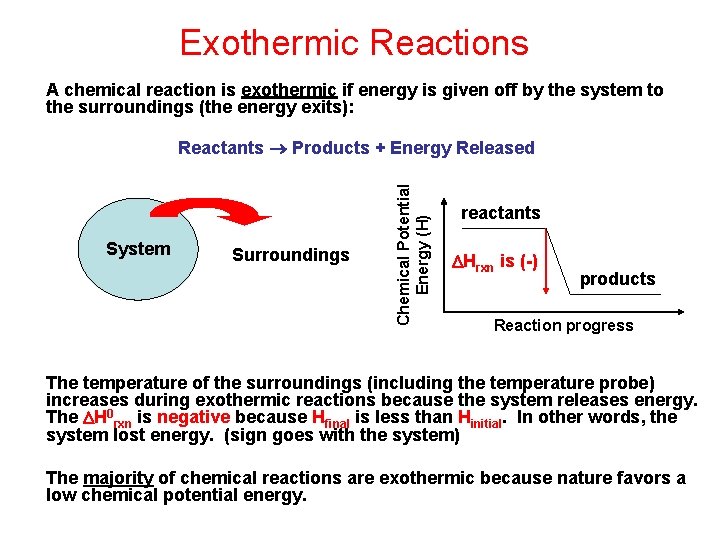
Exothermic Reactions A chemical reaction is exothermic if energy is given off by the system to the surroundings (the energy exits): System Surroundings Chemical Potential Energy (H) Reactants Products + Energy Released reactants Hrxn is (-) products Reaction progress The temperature of the surroundings (including the temperature probe) increases during exothermic reactions because the system releases energy. The H 0 rxn is negative because Hfinal is less than Hinitial. In other words, the system lost energy. (sign goes with the system) The majority of chemical reactions are exothermic because nature favors a low chemical potential energy.

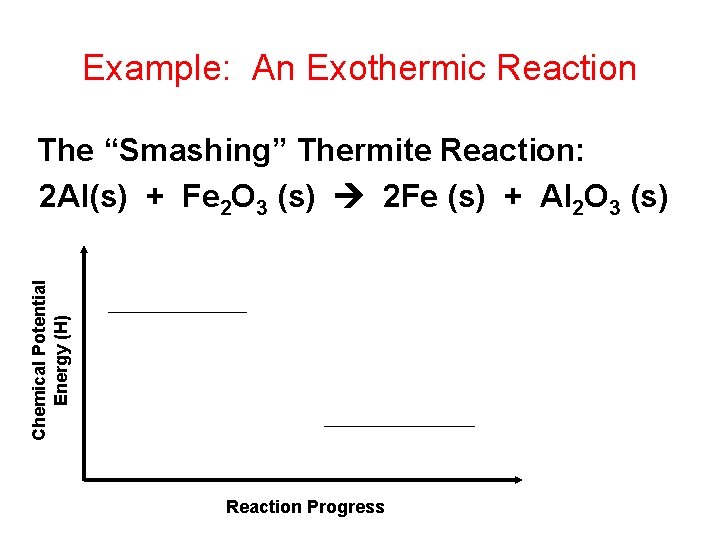
Example: An Exothermic Reaction Chemical Potential Energy (H) The “Smashing” Thermite Reaction: 2 Al(s) + Fe 2 O 3 (s) 2 Fe (s) + Al 2 O 3 (s) Reaction Progress

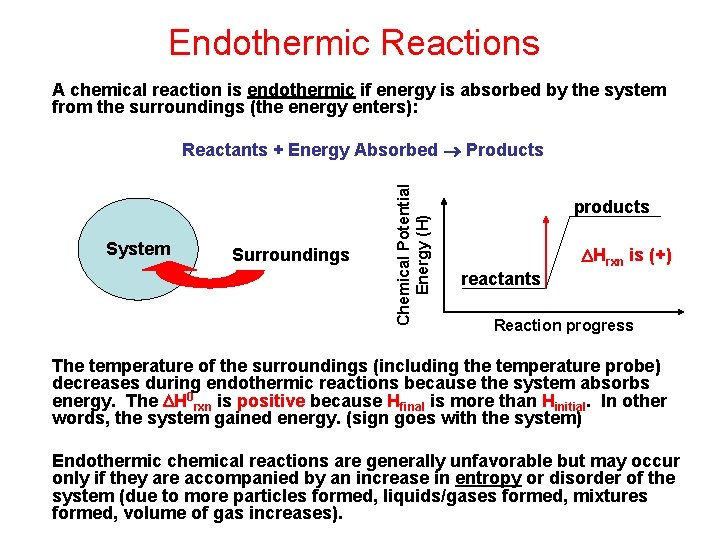
Endothermic Reactions A chemical reaction is endothermic if energy is absorbed by the system from the surroundings (the energy enters): System Surroundings Chemical Potential Energy (H) Reactants + Energy Absorbed Products products Hrxn is (+) reactants Reaction progress The temperature of the surroundings (including the temperature probe) decreases during endothermic reactions because the system absorbs energy. The H 0 rxn is positive because Hfinal is more than Hinitial. In other words, the system gained energy. (sign goes with the system) Endothermic chemical reactions are generally unfavorable but may occur only if they are accompanied by an increase in entropy or disorder of the system (due to more particles formed, liquids/gases formed, mixtures formed, volume of gas increases).

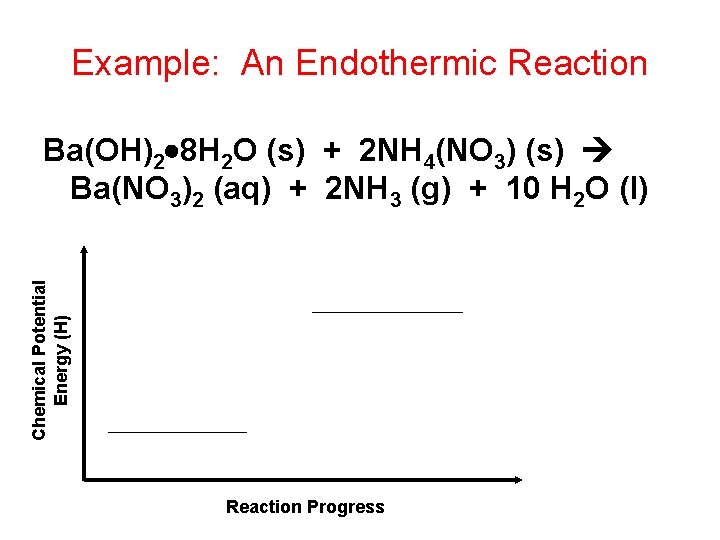
Example: An Endothermic Reaction Chemical Potential Energy (H) Ba(OH)2 8 H 2 O (s) + 2 NH 4(NO 3) (s) Ba(NO 3)2 (aq) + 2 NH 3 (g) + 10 H 2 O (l) Reaction Progress

Do you have to actually perform and observe a chemical reaction to know if it is exothermic or endothermic? • No – you can calculate H 0 rxn from data that has already been measured and tabulated by thermo -chemists (see handout). • H 0 f = standard heat of formation for a compound (in k. J/mol). It is determined by forming the compound from its elements in their stable forms at conditions of 298 K and 1 atm of pressure inside of a calorimeter. • For most compounds, H 0 f is negative because bond formation is exothermic! • H 0 f of an element is always 0 k. J/mol by def.

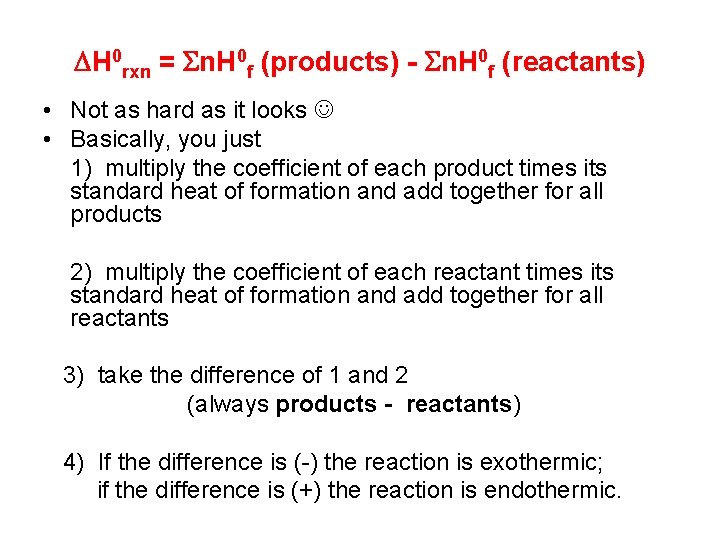
H 0 rxn = n. H 0 f (products) - n. H 0 f (reactants) • Not as hard as it looks • Basically, you just 1) multiply the coefficient of each product times its standard heat of formation and add together for all products 2) multiply the coefficient of each reactant times its standard heat of formation and add together for all reactants 3) take the difference of 1 and 2 (always products - reactants) 4) If the difference is (-) the reaction is exothermic; if the difference is (+) the reaction is endothermic.

Try this… • Calculate the H 0 rxn for thermite reaction using tabulated data (see handout): 2 Al (s) + Fe 2 O 3 (s) 2 Fe (s) + Al 2 O 3 (s) H 0 rxn = n. H 0 f (products) - n. H 0 f (reactants)

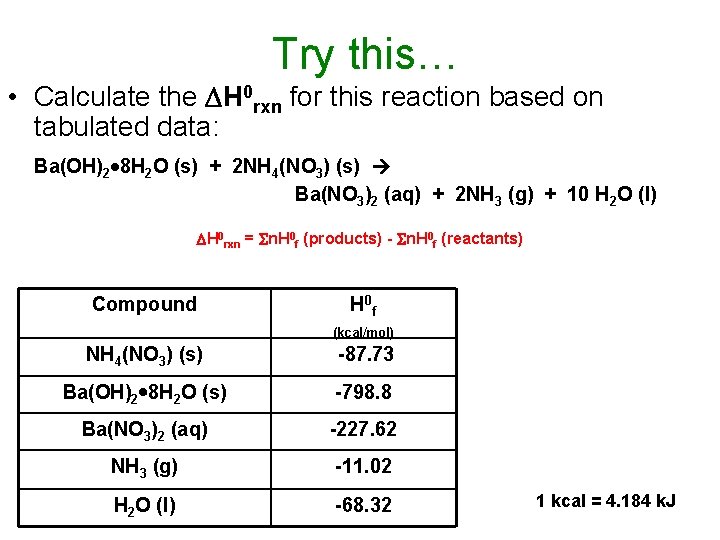
Try this… • Calculate the H 0 rxn for this reaction based on tabulated data: Ba(OH)2 8 H 2 O (s) + 2 NH 4(NO 3) (s) Ba(NO 3)2 (aq) + 2 NH 3 (g) + 10 H 2 O (l) H 0 rxn = n. H 0 f (products) - n. H 0 f (reactants) Compound H 0 f (kcal/mol) NH 4(NO 3) (s) -87. 73 Ba(OH)2 8 H 2 O (s) -798. 8 Ba(NO 3)2 (aq) -227. 62 NH 3 (g) -11. 02 H 2 O (l) -68. 32 1 kcal = 4. 184 k. J

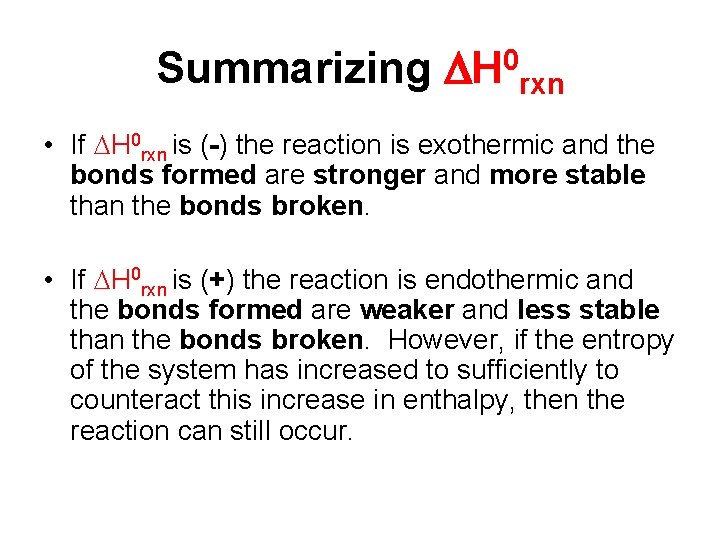
Summarizing H 0 rxn • If H 0 rxn is (-) the reaction is exothermic and the bonds formed are stronger and more stable than the bonds broken. • If H 0 rxn is (+) the reaction is endothermic and the bonds formed are weaker and less stable than the bonds broken. However, if the entropy of the system has increased to sufficiently to counteract this increase in enthalpy, then the reaction can still occur.

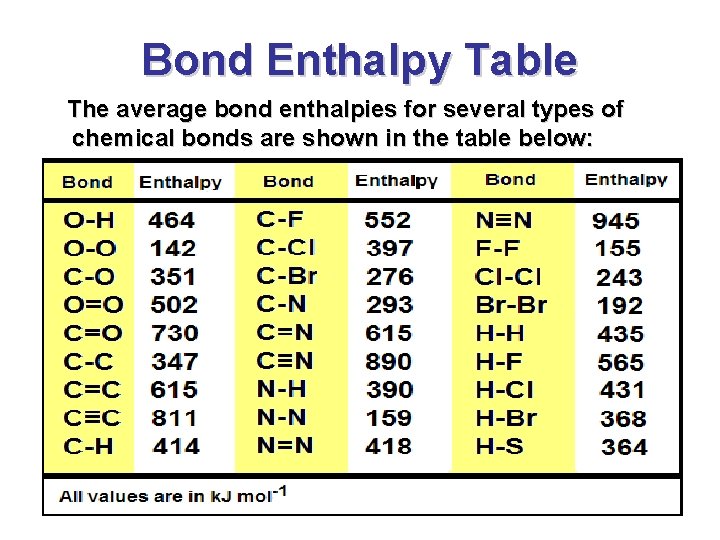
Bond Enthalpies • Another way to determining an enthalpy change ( H 0 rxn) for a chemical reaction is to compute the difference in bond enthalpies between reactants and products • The energy to required to break a covalent bond in the gaseous phase is called a bond enthalpy (bond dissociation energy). • Bond enthalpy tables give the average energy to break a chemical bond. Actually there are slight variations depending on the environment in which the chemical bond is located

Bond Enthalpy Table The average bond enthalpies for several types of chemical bonds are shown in the table below:

Bond Enthalpies • Bond enthalpies can be used to calculate the enthalpy change ( H 0 rxn) for a chemical reaction. • Energy is required to break chemical bonds. Therefore when a chemical bond is broken its enthalpy change carries a positive sign. • Energy is released when chemical bonds form. When a chemical bond is formed its enthalpy change is expressed as a negative value. • By combining the enthalpy required and the enthalpy released for the breaking and forming chemical bonds, one can calculate the overall enthalpy change for a chemical reaction.

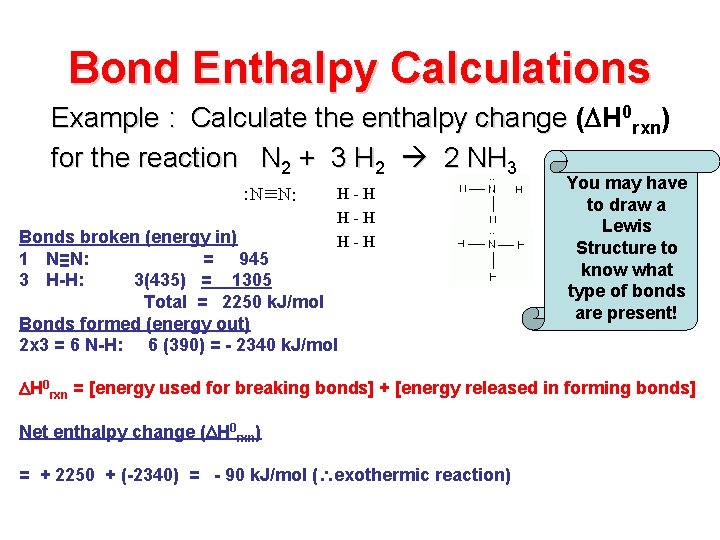
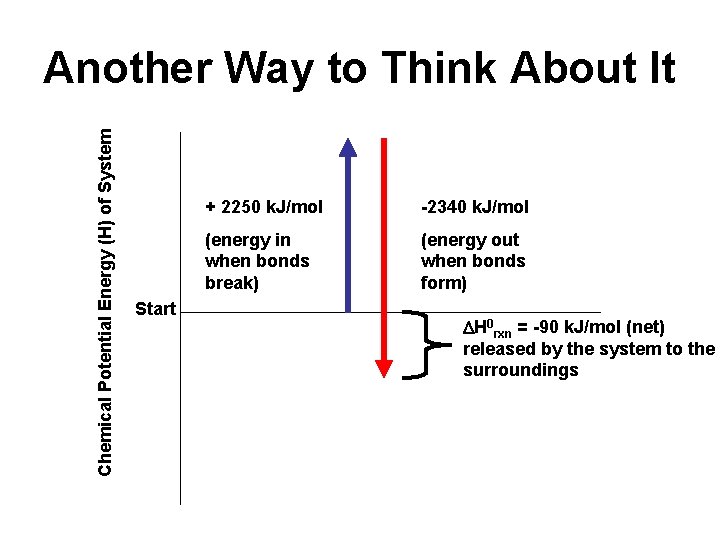
Bond Enthalpy Calculations Example : Calculate the enthalpy change ( H 0 rxn) for the reaction N 2 + 3 H 2 2 NH 3 H-H H-H Bonds broken (energy in) 1 N≡N: = 945 3 H-H: 3(435) = 1305 Total = 2250 k. J/mol Bonds formed (energy out) 2 x 3 = 6 N-H: 6 (390) = - 2340 k. J/mol You may have to draw a Lewis Structure to know what type of bonds are present! H 0 rxn = [energy used for breaking bonds] + [energy released in forming bonds] Net enthalpy change ( H 0 rxn) = + 2250 + (-2340) = - 90 k. J/mol ( exothermic reaction)

Chemical Potential Energy (H) of System Another Way to Think About It Start + 2250 k. J/mol -2340 k. J/mol (energy in when bonds break) (energy out when bonds form) H 0 rxn = -90 k. J/mol (net) released by the system to the surroundings