Introduction to Chemical Reactions Making new substances Targets

Introduction to Chemical Reactions Making new substances

Targets I can… Represent a chemical reaction by writing a Chemical Equations. Balance Chemical Equations to show the same number of atoms of each element on each side. Explain that a balanced chemical equation obeys The Law of Conservation of Mass (saying that atoms won’t be created or destroyed in a chemical reaction. ) Analyze a chemical equation. Identify the 5 basic types of chemical reactions, reactants, products Identify signs of a chemical reaction from lab observations

How do you know when a chemical reaction takes place? Color Change Precipitate Formation

How do you know when a chemical reaction takes place? Gas Formation Odor

How do you know when a chemical reaction takes place? Temperature Change (energy change) exothermic endothermic

How do you know when a chemical reaction takes place? Produce light (energy change) Change in Acidity
Representing Chemical Reactions Chemists observe chemical reactions and have come up with a shorthand way to represent or model what is happening. Word equations are written as chemical equations by replacing chemical names with correct chemical formulas Solid Sodium combines with Chlorine gas to make solid Sodium Chloride: 2 Na (s) + Cl 2 (g) 2 Na. Cl

Chemical Equations Chemical Equation 2 Na + Cl 2 2 Na. Cl Reactants – starting substances in chemical reaction, written on left side of arrow Products – substance(s) formed from the reaction, written on right side of arrow Reactant Product When more than one reactant or product they are separated with a plus (+) sign The arrow shows the direction of the reaction
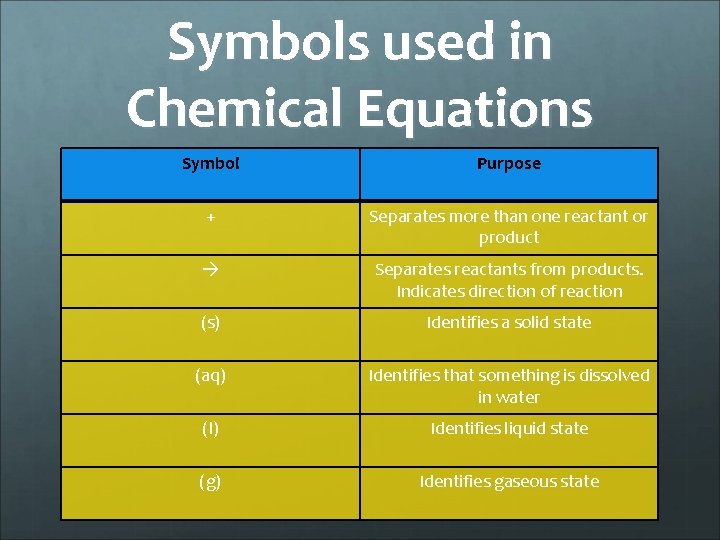
Symbols used in Chemical Equations Symbol Purpose + Separates more than one reactant or product Separates reactants from products. Indicates direction of reaction (s) Identifies a solid state (aq) Identifies that something is dissolved in water (l) Identifies liquid state (g) Identifies gaseous state

Law of Conservation of Mass In a chemical reaction, matter is neither created nor destroyed. Atoms won’t change their identity (e. g. a Carbon atom can’t become an Iron atom) This means that you have to have the same number of each type of atom on each side of the chemical equation. Conservation of Mass Video

Balancing Equations After you write a chemical equation you have to balance it to make sure that the same number of atoms of each element are on each side. (law of conservation of mass)
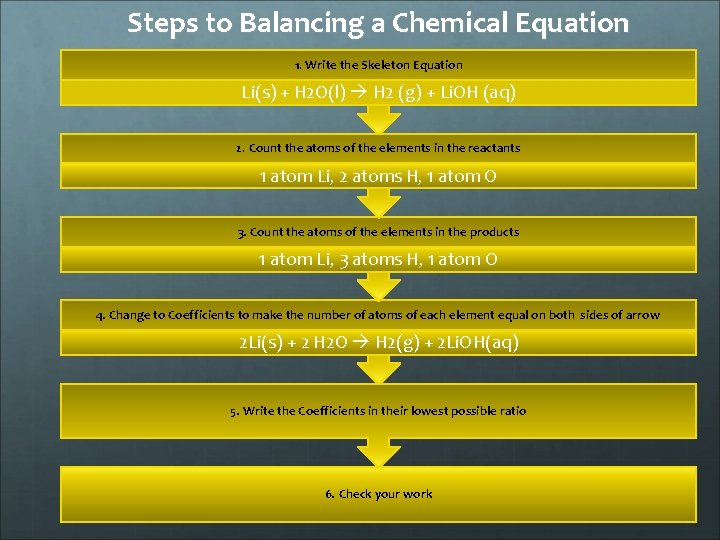
Steps to Balancing a Chemical Equation 1. Write the Skeleton Equation Li(s) + H 2 O(l) H 2 (g) + Li. OH (aq) 2. Count the atoms of the elements in the reactants 1 atom Li, 2 atoms H, 1 atom O 3. Count the atoms of the elements in the products 1 atom Li, 3 atoms H, 1 atom O 4. Change to Coefficients to make the number of atoms of each element equal on both sides of arrow 2 Li(s) + 2 H 2 O H 2(g) + 2 Li. OH(aq) 5. Write the Coefficients in their lowest possible ratio 6. Check your work
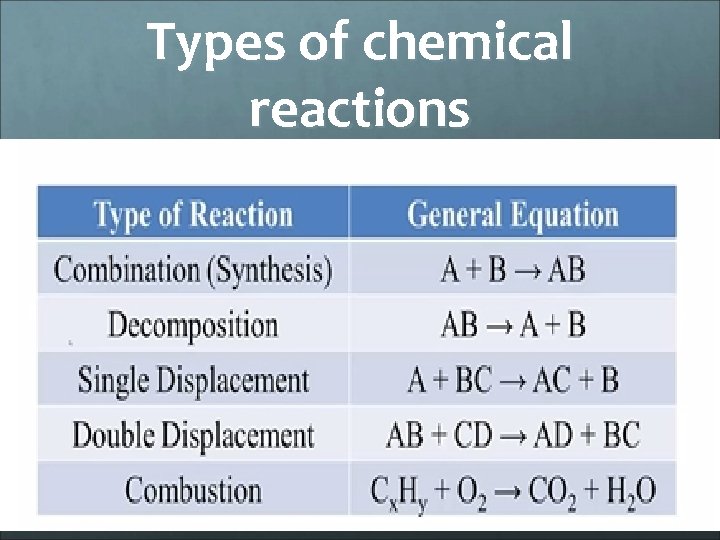
Types of chemical reactions
Types of chemical reactions Combination – two or more substances combine to form one product Decomposition – one substance breaks down into two or more simpler substances Single replacement – one single element replaces another Double replacement – two compounds switch cations. Products can be insoluble in water(precipitate) Combustion - substance (hydrocarbon) combines with oxygen to produce carbon dioxide and water and energy (exothermic)
- Slides: 14