Introduction to Chemical Reactions Chemical Equations I II



- Slides: 15

Introduction to Chemical Reactions Chemical Equations I II IV V

Signs of a Chemical Reaction • • Evolution of heat and light Formation of a gas Formation of a precipitate Color change

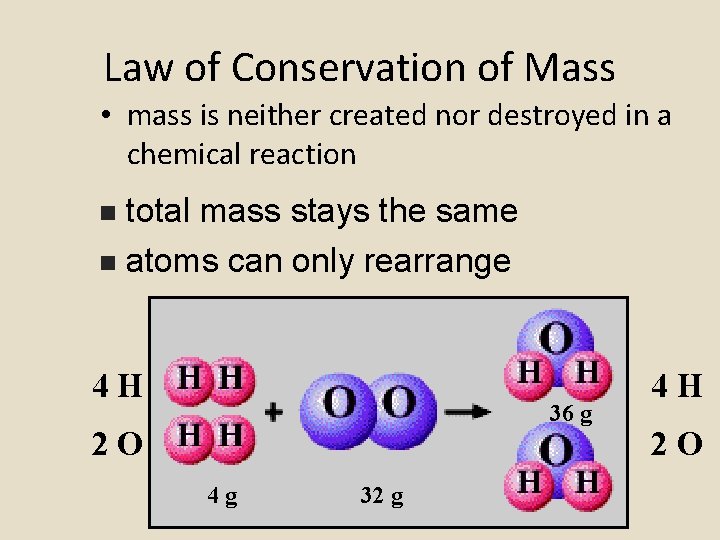
Law of Conservation of Mass • mass is neither created nor destroyed in a chemical reaction total mass stays the same n atoms can only rearrange n 4 H 36 g 2 O 4 g 32 g 4 H 2 O

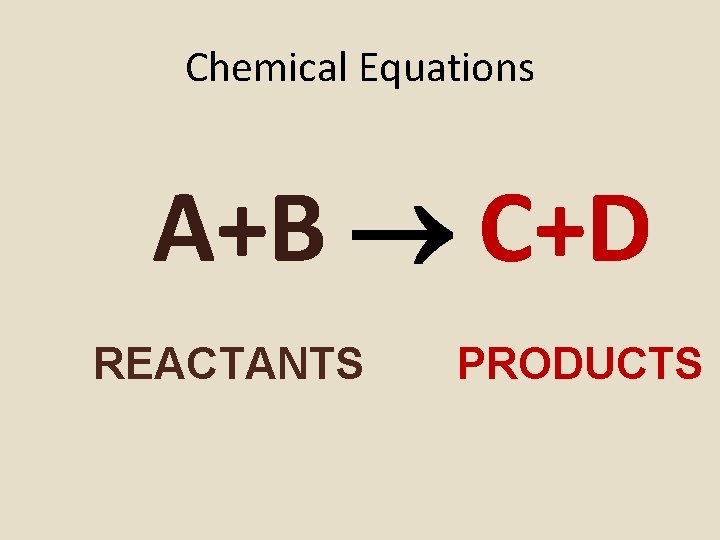
Chemical Equations A+B C+D REACTANTS PRODUCTS

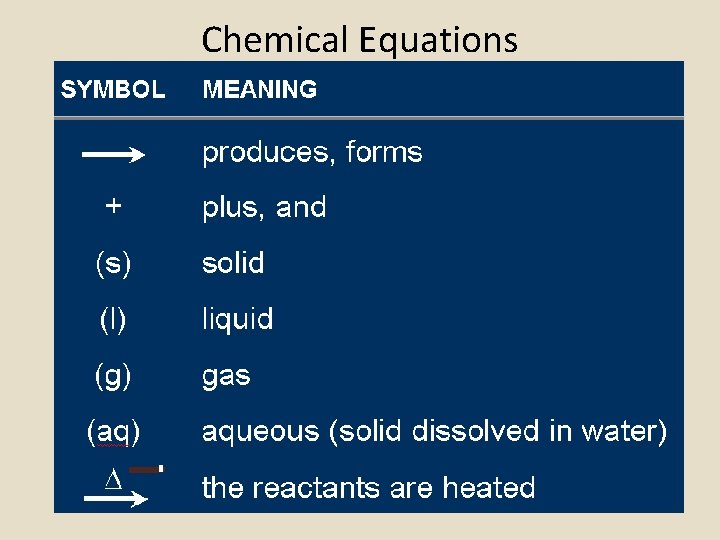
Chemical Equations

Writing Equations 2 H 2(g) + O 2(g) 2 H 2 O(g) • Identify the substances involved. • Use symbols to show: · How many? - coefficient · Of what? - chemical formula · In what state? - physical state n Remember the diatomic elements.

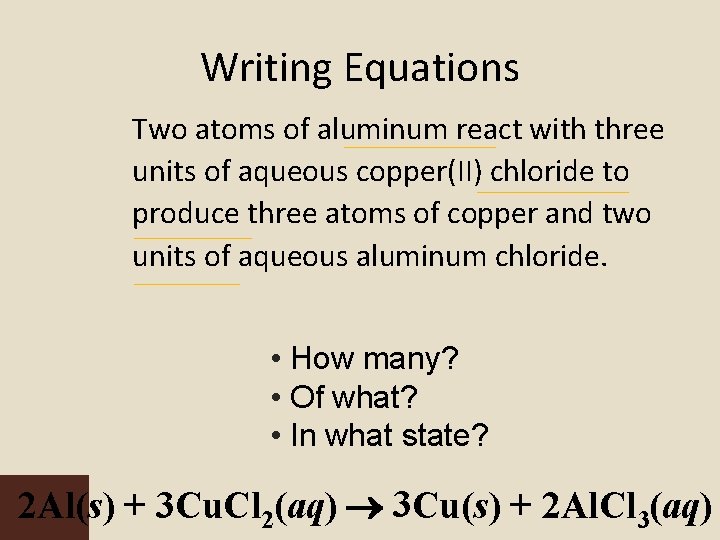
Writing Equations Two atoms of aluminum react with three units of aqueous copper(II) chloride to produce three atoms of copper and two units of aqueous aluminum chloride. • How many? • Of what? • In what state? 2 Al(s) + 3 Cu. Cl 2(aq) 3 Cu(s) + 2 Al. Cl 3(aq)

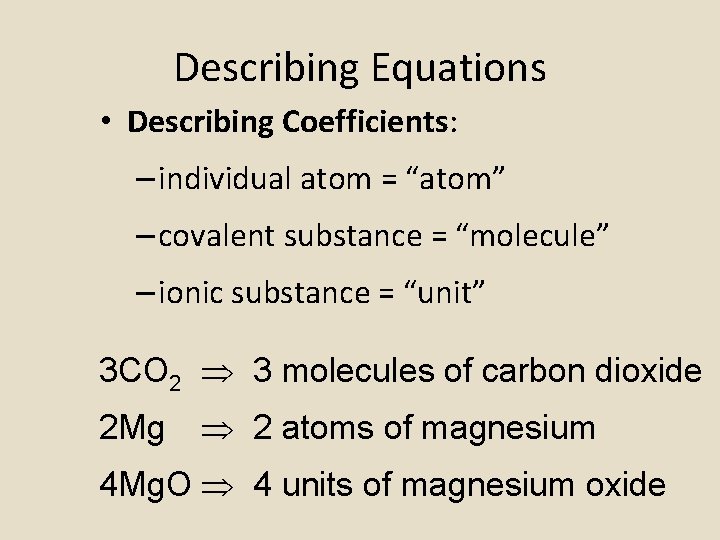
Describing Equations • Describing Coefficients: – individual atom = “atom” – covalent substance = “molecule” – ionic substance = “unit” 3 CO 2 3 molecules of carbon dioxide 2 Mg 2 atoms of magnesium 4 Mg. O 4 units of magnesium oxide

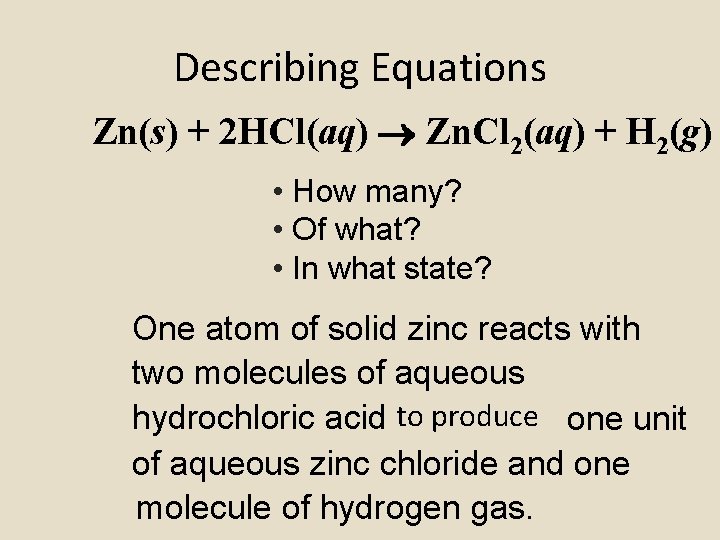
Describing Equations Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g) • How many? • Of what? • In what state? One atom of solid zinc reacts with two molecules of aqueous hydrochloric acid to produce one unit of aqueous zinc chloride and one molecule of hydrogen gas.

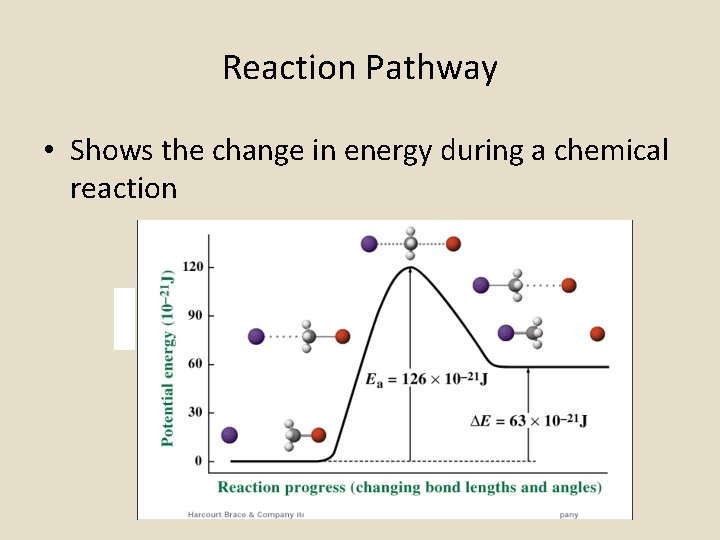
Reaction Pathway • Shows the change in energy during a chemical reaction

Balancing Steps 1. Write the unbalanced equation. 2. Count atoms on each side. 3. Use coefficients to make #s equal. Coefficient subscript = # of atoms 4. Reduce coefficients to lowest possible ratio, if necessary. 5. Double check atom balance!!!

Helpful Tips • Balance one element at a time. • Update ALL atom counts after adding a coefficient. • If an element appears more than once per side, balance it last. • Balance polyatomic ions as single units. – “ 1 SO 4” instead of “ 1 S” and “ 4 O” C. Johannesson

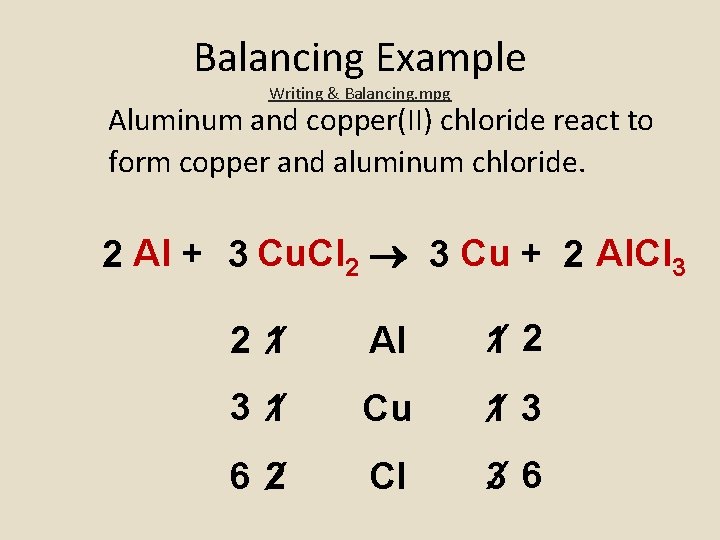
Balancing Example Writing & Balancing. mpg Aluminum and copper(II) chloride react to form copper and aluminum chloride. 2 Al + 3 Cu. Cl 2 3 Cu + 2 Al. Cl 3 2 1 Al 1 2 3 1 Cu 1 3 6 2 Cl 3 6

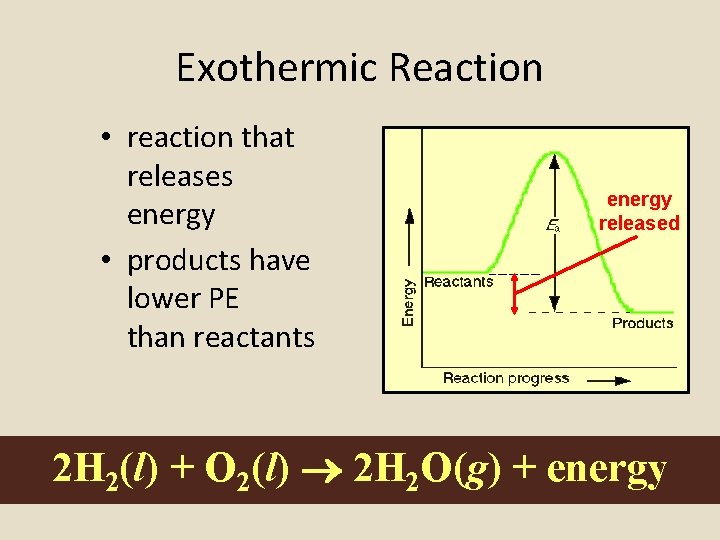
Exothermic Reaction • reaction that releases energy • products have lower PE than reactants energy released 2 H 2(l) + O 2(l) 2 H 2 O(g) + energy

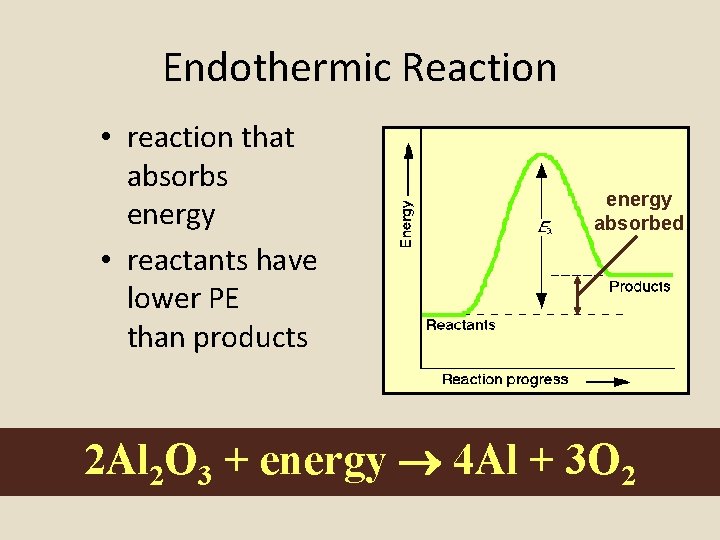
Endothermic Reaction • reaction that absorbs energy • reactants have lower PE than products energy absorbed 2 Al 2 O 3 + energy 4 Al + 3 O 2