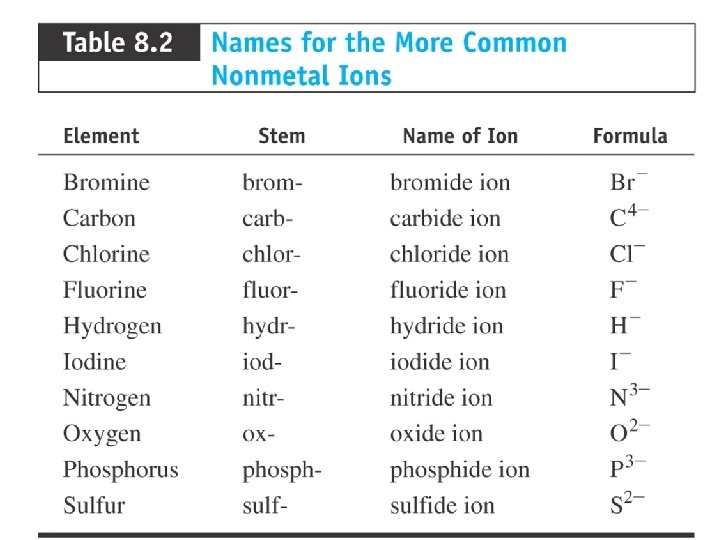
Introduction to Chemical Principles Chapter 8 Chemical Nomenclature














- Slides: 38

Introduction to Chemical Principles Chapter 8: Chemical Nomenclature Gilbert Lewis

Nomenclature Chemical Nomenclature is the system of names used to distinguish compounds from each other and the rules needed to device these names. We will consider how to name: – Binary ionic compounds, which contain a metal and a nonmetal. – Binary molecular compounds, which consist only of nonmetals. (For naming purposes metalloids are treated as nonmetals) – Binary and oxo-acids, compounds that release hydrogen ions when dissolved in water.

Classifying Elements & Compounds resulting from the combination of a metal and one or nore nonmetal are called ionic Compounds resulting from the combination of a nonmetal with other nonmetal are considered molecule. A binary compound is a compound in which only two element are present A binary ionic compound is an ionic compound in which one element present is a metal and the other element present is a non metal. The metal is always present as the positive ion, and the nonmetal is always present as the negative ion.

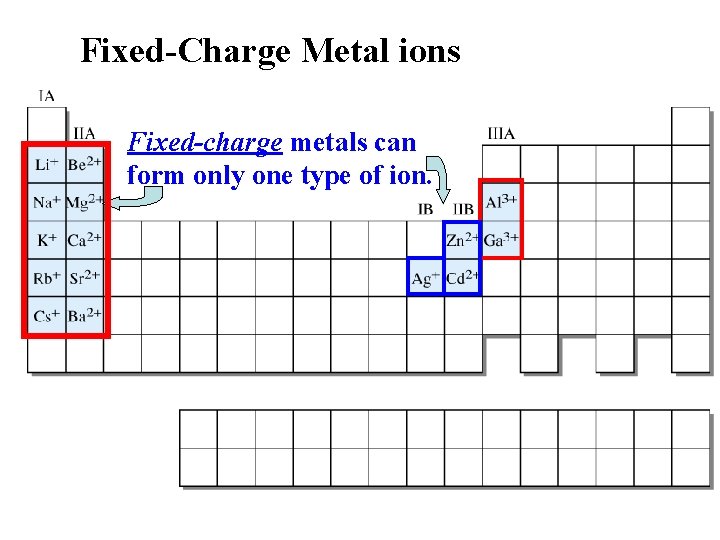
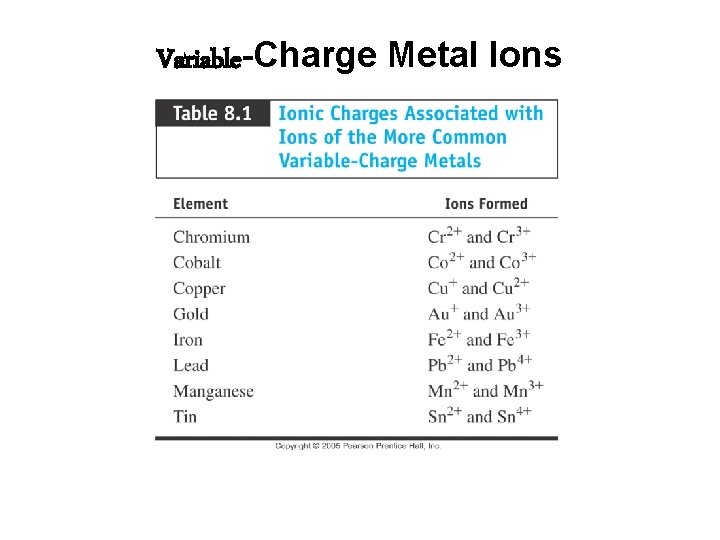
For nomenclature purposes, there are two types of metal ions: Fixed Charge metal ions only form one type of positive ion – always has the same charge. Variable Charge metal ions can form more than one type of positive ion, with the ion type differing in charge and magnitude.

Fixed-Charge Metal ions Fixed-charge metals can form only one type of ion.

Variable-Charge Metal Ions • Which metals have a variable charge? The rest of them!

Variable-Charge Metal Ions

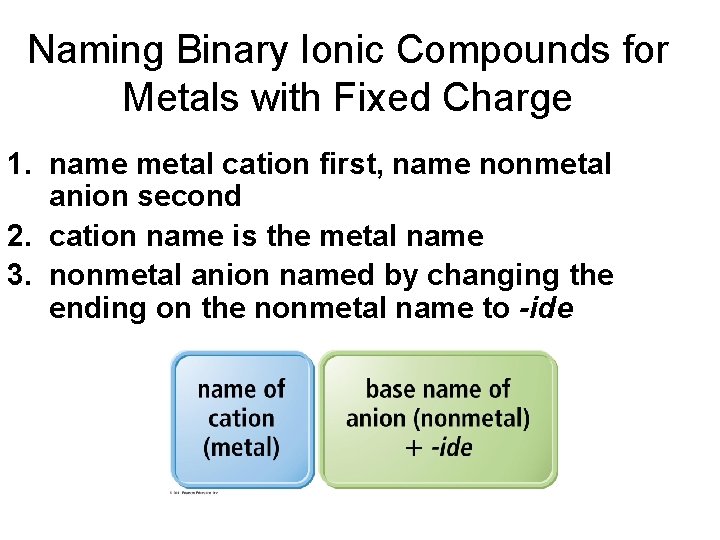
Naming Binary Ionic Compounds for Metals with Fixed Charge 1. name metal cation first, name nonmetal anion second 2. cation name is the metal name 3. nonmetal anion named by changing the ending on the nonmetal name to -ide

Example: Naming Binary Ionic with Fixed Charge Metal Cs. F 1. Identify cation and anion Cs = Cs+ because it is Group 1 A F = F− because it is Group 7 A 2. Name the cation Cs+ = cesium 3. Name the anion F− = fluoride 4. Write the cation name first, then the anion name cesium fluoride


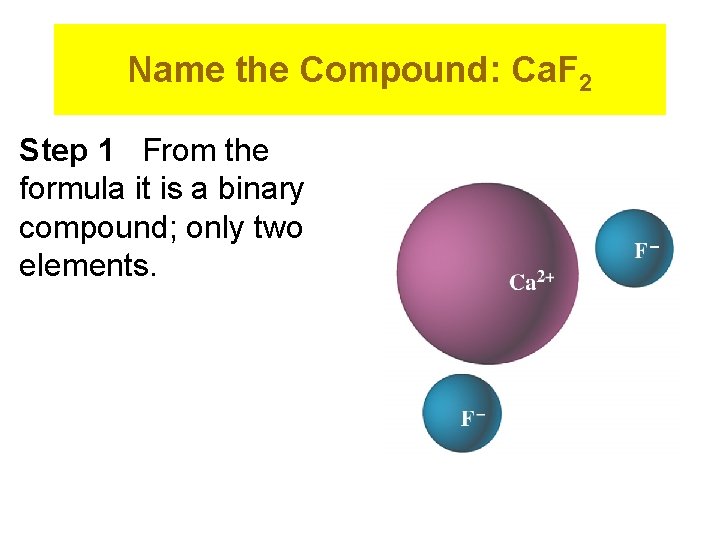
Name the Compound: Ca. F 2 Step 1 From the formula it is a binary compound; only two elements.

12

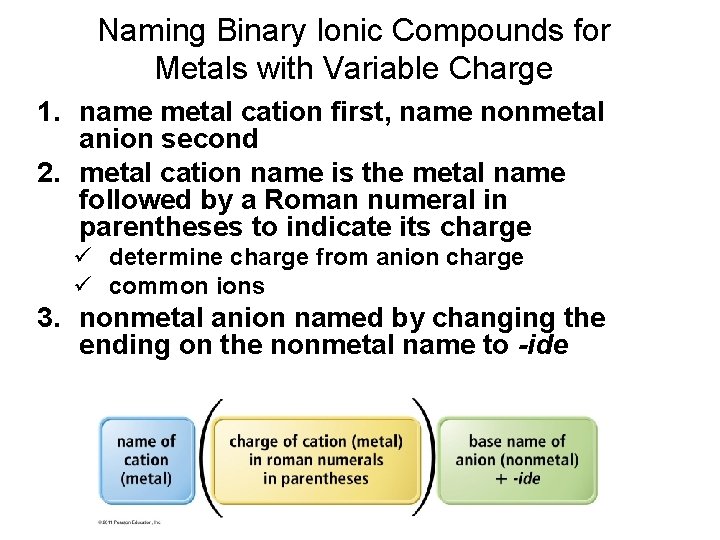
Naming Binary Ionic Compounds for Metals with Variable Charge 1. name metal cation first, name nonmetal anion second 2. metal cation name is the metal name followed by a Roman numeral in parentheses to indicate its charge ü determine charge from anion charge ü common ions 3. nonmetal anion named by changing the ending on the nonmetal name to -ide

Practice — Find the charge on the cation 1. Ti. Cl 4 4 Cl = 4−, Ti = 4+ 2. Cr. O 3 3 O = 6−, Cr = 6+ 3. Fe 3 N 2 2 N = 6−, 3 Fe = 6+, Fe = 2+

Example: Naming binary ionic with variable charge metal Cu. F 2 1. Identify cation and anion F = F− because it is Group 7 Cu = Cu 2+ to balance the two (−) charges from 2 F− 2. Name the cation Cu 2+ = copper(II) 3. Name the anion F− = fluoride 4. Write the cation name first, then the anion name copper(II) fluoride 15 Tro: Chemistry: A Molecular Approach,

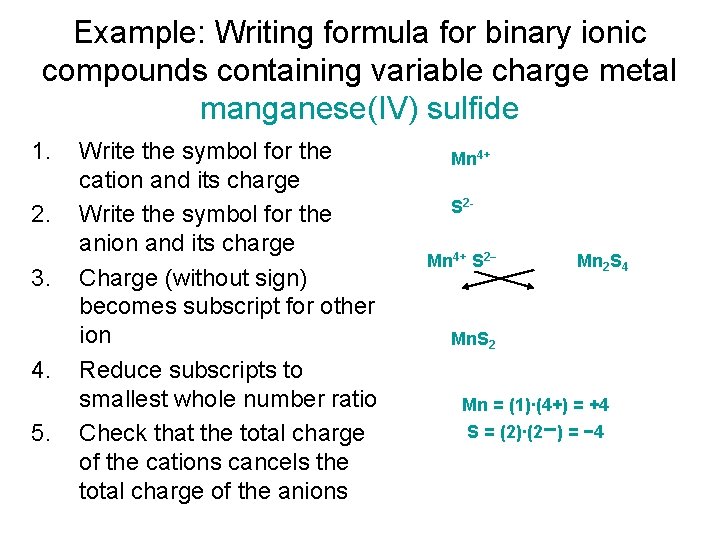
Example: Writing formula for binary ionic compounds containing variable charge metal manganese(IV) sulfide 1. 2. 3. 4. 5. Write the symbol for the cation and its charge Write the symbol for the anion and its charge Charge (without sign) becomes subscript for other ion Reduce subscripts to smallest whole number ratio Check that the total charge of the cations cancels the total charge of the anions Mn 4+ S 2− Mn 2 S 4 Mn. S 2 Mn = (1)∙(4+) = +4 S = (2)∙(2−) = − 4

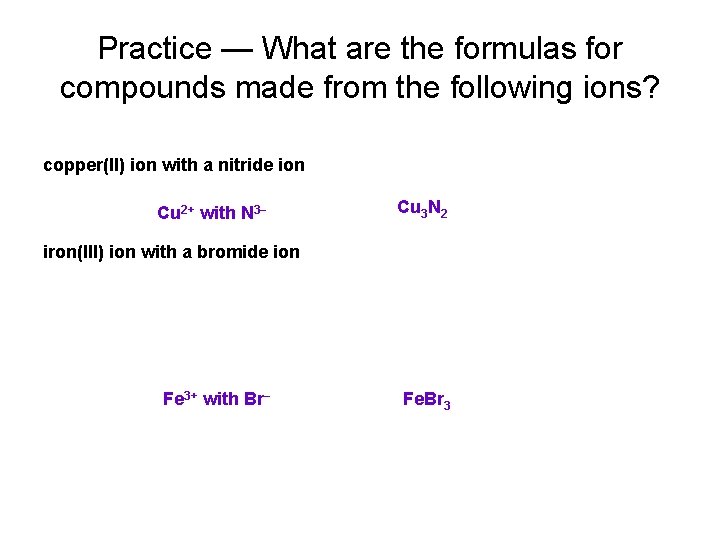
Practice — What are the formulas for compounds made from the following ions? copper(II) ion with a nitride ion Cu 2+ with N 3− Cu 3 N 2 iron(III) ion with a bromide ion Fe 3+ with Br− Fe. Br 3

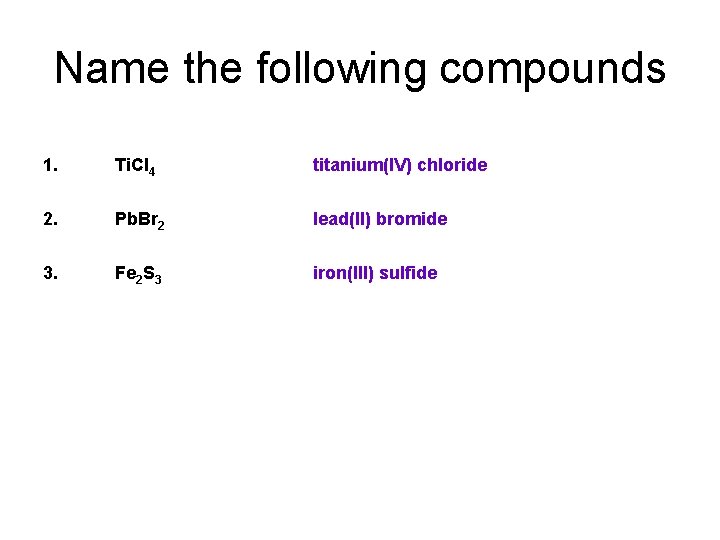
Name the following compounds 1. Ti. Cl 4 titanium(IV) chloride 2. Pb. Br 2 lead(II) bromide 3. Fe 2 S 3 iron(III) sulfide

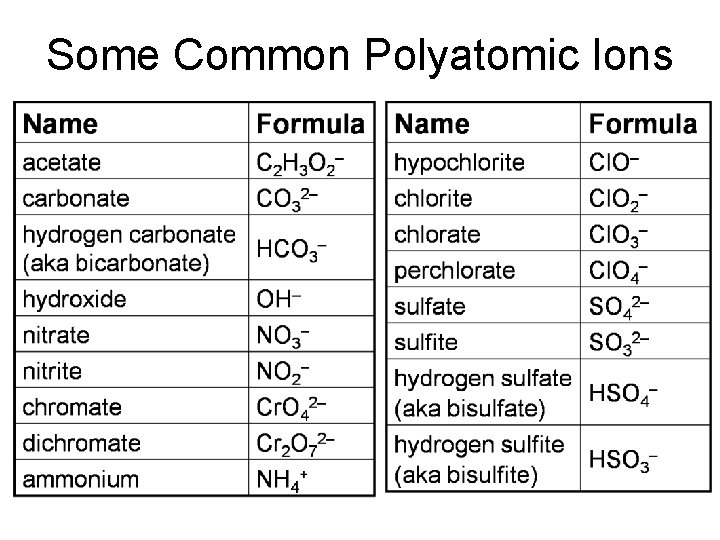
Some Common Polyatomic Ions

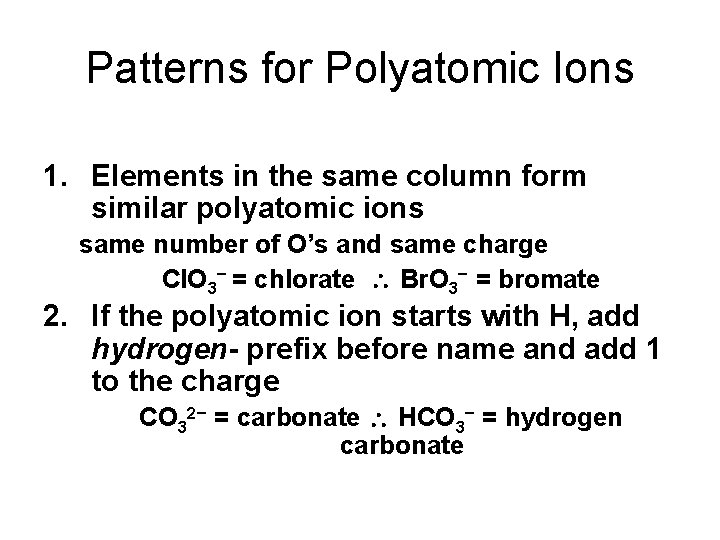
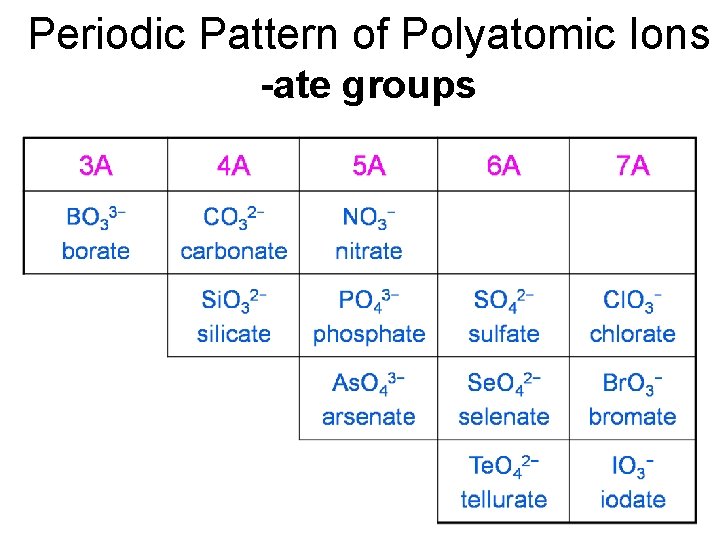
Patterns for Polyatomic Ions 1. Elements in the same column form similar polyatomic ions same number of O’s and same charge Cl. O 3− = chlorate Br. O 3− = bromate 2. If the polyatomic ion starts with H, add hydrogen- prefix before name and add 1 to the charge CO 32− = carbonate HCO 3− = hydrogen carbonate

Periodic Pattern of Polyatomic Ions -ate groups

Example: Naming ionic compounds containing a polyatomic ion Na 2 SO 4 1. Identify the ions Na = Na+ because in Group 1 A SO 4 = SO 42− a polyatomic ion 2. Name the cation Na+ = sodium, metal with invariant charge 3. Name the anion SO 42− = sulfate 4. Write the name of the cation followed by the name of the anion sodium sulfate

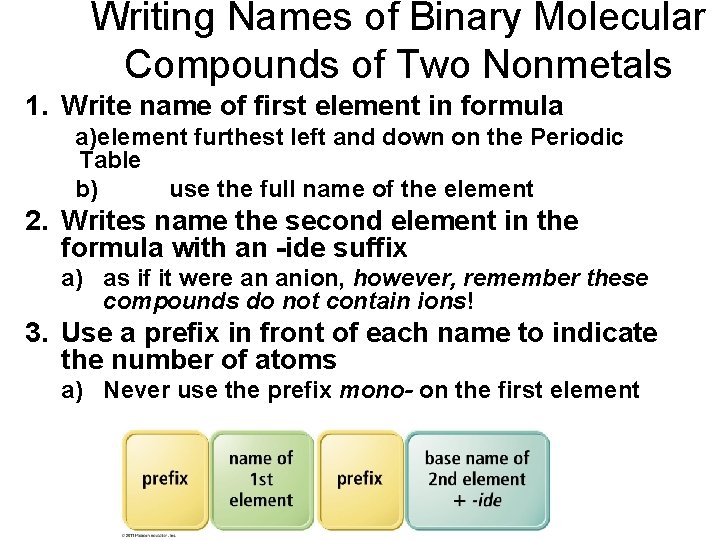
Writing Names of Binary Molecular Compounds of Two Nonmetals 1. Write name of first element in formula a)element furthest left and down on the Periodic Table b) use the full name of the element 2. Writes name the second element in the formula with an -ide suffix a) as if it were an anion, however, remember these compounds do not contain ions! 3. Use a prefix in front of each name to indicate the number of atoms a) Never use the prefix mono- on the first element

Subscript – Prefixes • 1 = monoü not used on first nonmetal • • 2 = di 3 = tri 4 = tetra 5 = penta- • Drop last “a” if name begins with a vowel • 6 = hexa • • 7 = hepta 8 = octa 9 = nona 10 = deca-

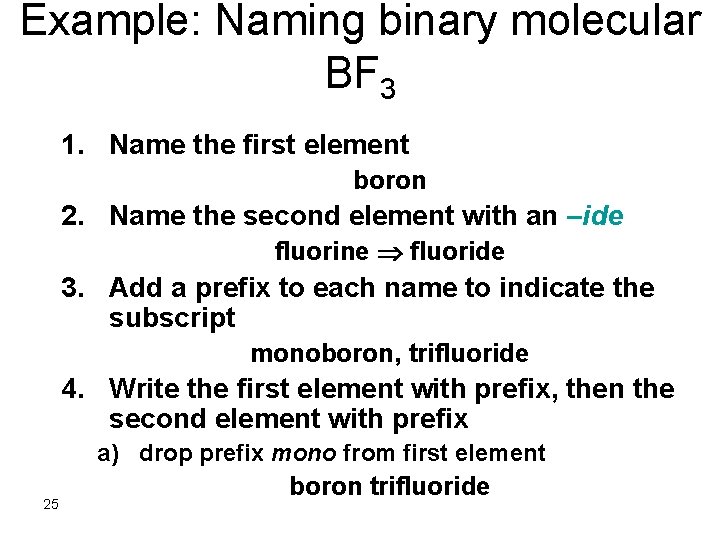
Example: Naming binary molecular BF 3 1. Name the first element boron 2. Name the second element with an –ide fluorine fluoride 3. Add a prefix to each name to indicate the subscript monoboron, trifluoride 4. Write the first element with prefix, then the second element with prefix a) drop prefix mono from first element 25 boron trifluoride

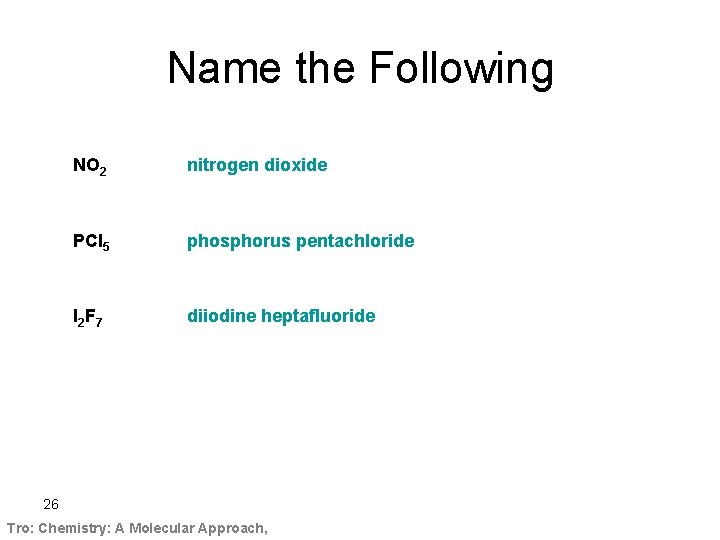
Name the Following NO 2 nitrogen dioxide PCl 5 phosphorus pentachloride I 2 F 7 diiodine heptafluoride 26 Tro: Chemistry: A Molecular Approach,

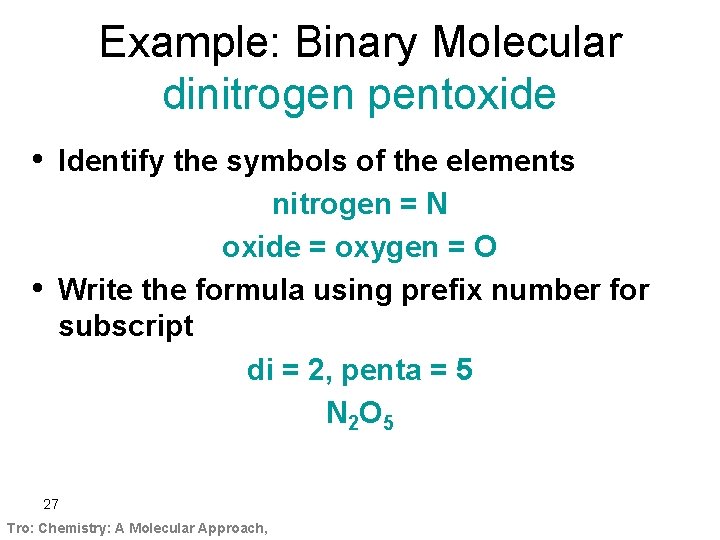
Example: Binary Molecular dinitrogen pentoxide • Identify the symbols of the elements • nitrogen = N oxide = oxygen = O Write the formula using prefix number for subscript di = 2, penta = 5 N 2 O 5 27 Tro: Chemistry: A Molecular Approach,

Write Formulas for the Following 28 dinitrogen tetroxide N 2 O 4 sulfur hexafluoride SF 6 diarsenic trisulfide As 2 S 3

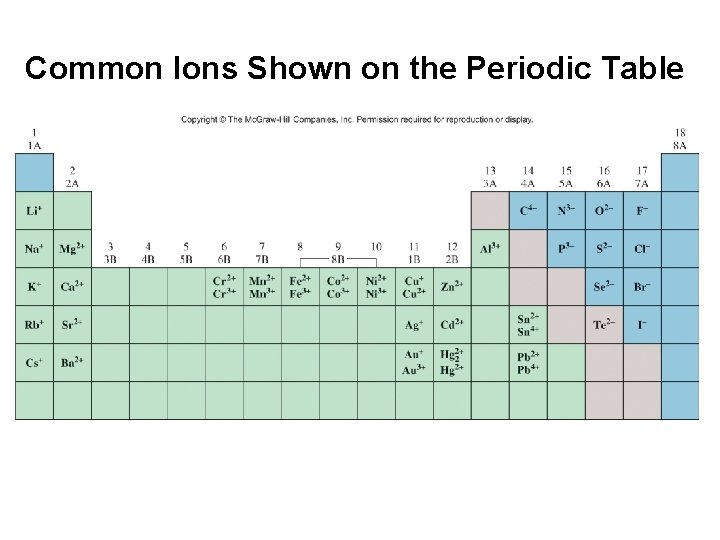
Common Ions Shown on the Periodic Table

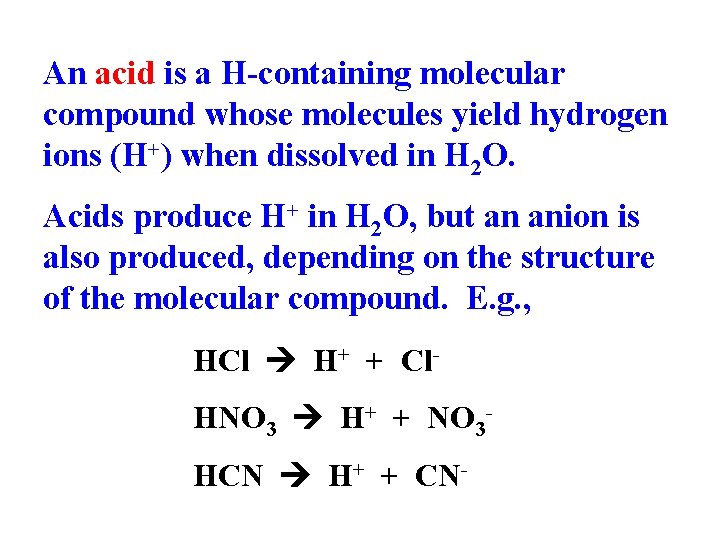
An acid is a H-containing molecular compound whose molecules yield hydrogen ions (H+) when dissolved in H 2 O. Acids produce H+ in H 2 O, but an anion is also produced, depending on the structure of the molecular compound. E. g. , HCl H+ + Cl. HNO 3 H+ + NO 3 HCN H+ + CN-

Acids • If the anion does not contain oxygen, the acid is named with the prefix hydro– and the suffix –ic. • Examples: HCl Hydrochloric acid HCN Hydrocyanic acid H 2 S Hydrosulfuric acid 31

Acids • If the anion does contain oxygen: – The suffix –ic is added to the root name if the anion name ends in –ate. • Examples: HNO 3 Nitric acid H 2 SO 4 Sulfuric acid HC 2 H 3 O 2 Acetic acid 32

Acids • If the anion does contain oxygen: – The suffix –ous is added to the root name if the anion name ends in –ite. • Examples: HNO 2 Nitrous acid H 2 SO 3 Sulfurous acid HCl. O 2 Chlorous acid 33

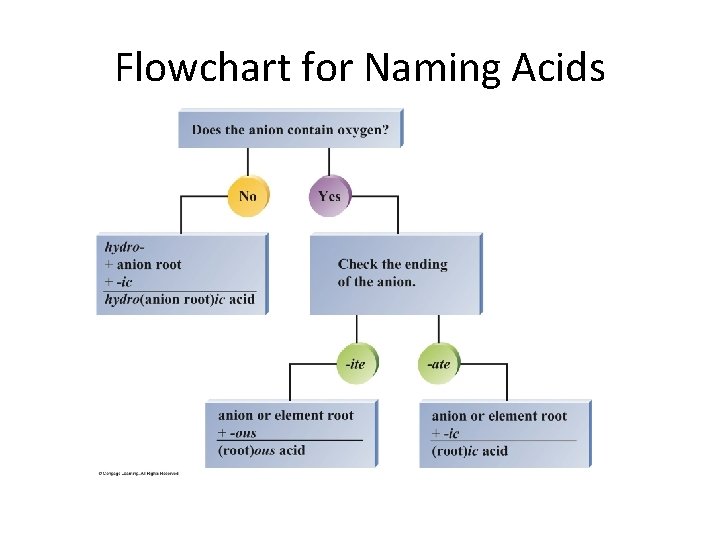
Flowchart for Naming Acids

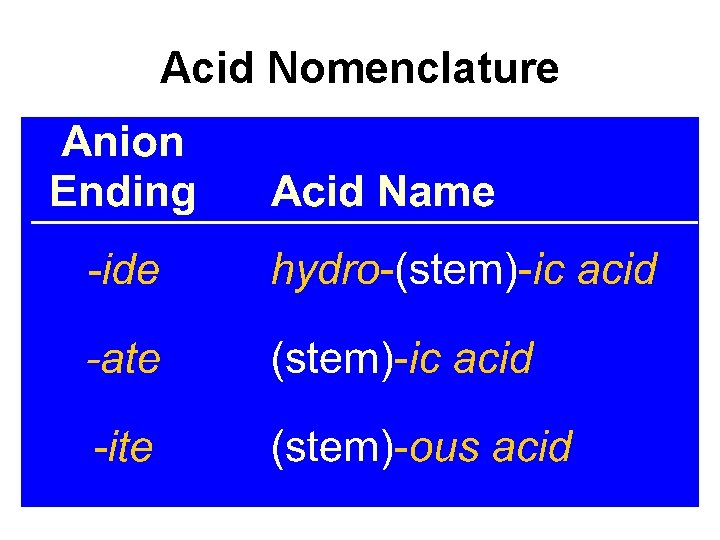
Acid Nomenclature

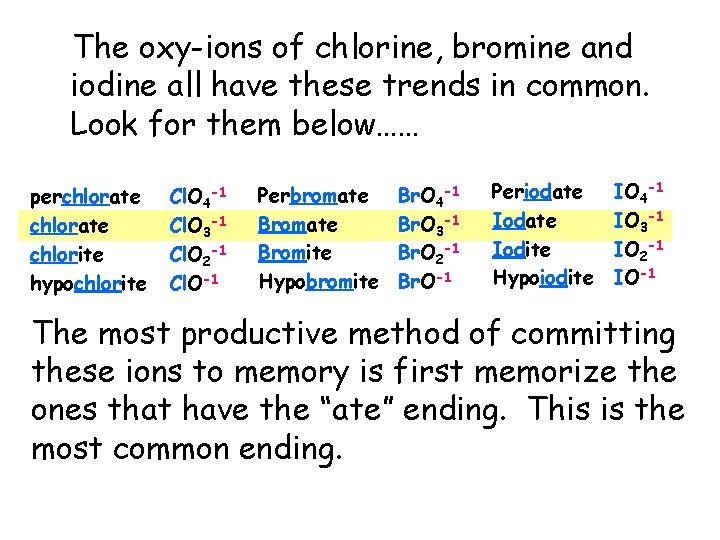
The oxy-ions of chlorine, bromine and iodine all have these trends in common. Look for them below…… perchlorate chlorite hypochlorite Cl. O 4 -1 Cl. O 3 -1 Cl. O 2 -1 Cl. O-1 Perbromate Bromite Hypobromite Br. O 4 -1 Br. O 3 -1 Br. O 2 -1 Br. O-1 Periodate Iodite Hypoiodite IO 4 -1 IO 3 -1 IO 2 -1 IO-1 The most productive method of committing these ions to memory is first memorize the ones that have the “ate” ending. This is the most common ending.

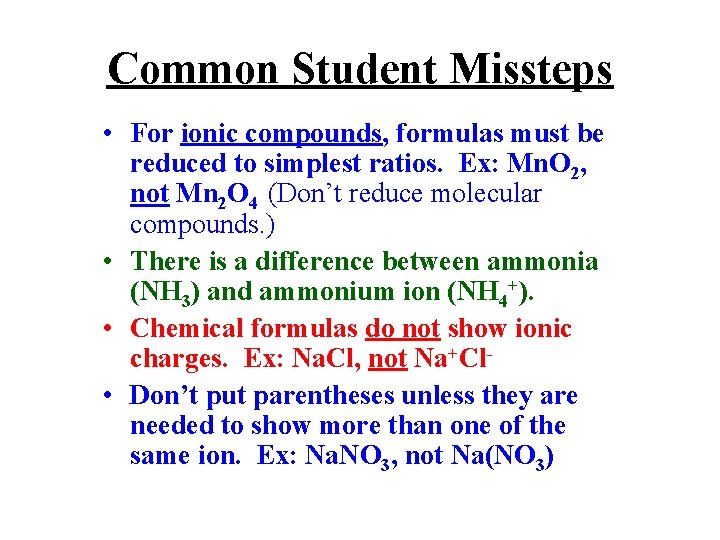
Common Student Missteps • For ionic compounds, formulas must be reduced to simplest ratios. Ex: Mn. O 2, not Mn 2 O 4 (Don’t reduce molecular compounds. ) • There is a difference between ammonia (NH 3) and ammonium ion (NH 4+). • Chemical formulas do not show ionic charges. Ex: Na. Cl, not Na+Cl • Don’t put parentheses unless they are needed to show more than one of the same ion. Ex: Na. NO 3, not Na(NO 3)

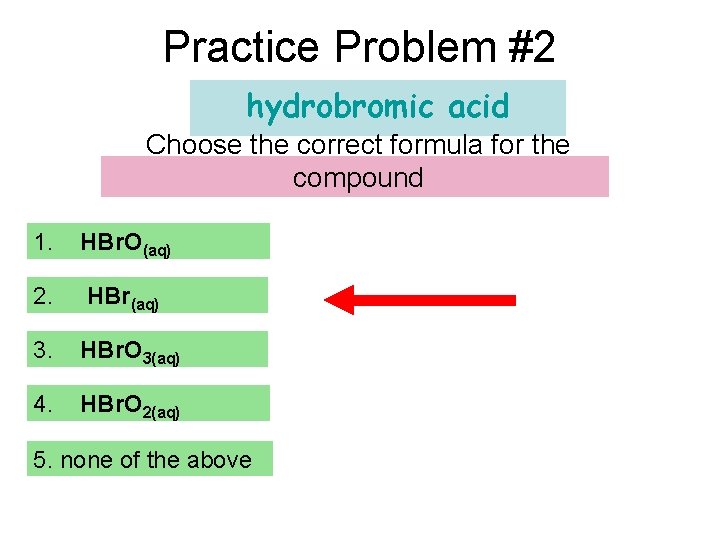
Practice Problem #2 hydrobromic acid Choose the correct formula for the compound 1. HBr. O(aq) 2. HBr(aq) 3. HBr. O 3(aq) 4. HBr. O 2(aq) 5. none of the above