INTRODUCTION TO BONDING AND ELECTRON DOTLEWIS STRUCTURES Chemistry
INTRODUCTION TO BONDING AND ELECTRON DOT/LEWIS STRUCTURES Chemistry
Why is bonding important? ? Without bonding the universe would be a mass of individual atoms. The type of bond defines the properties of a substance. Na. Cl…shape H 2 O and oil…dissolving

Electronegativity Ability of atoms within a molecule to attract electrons
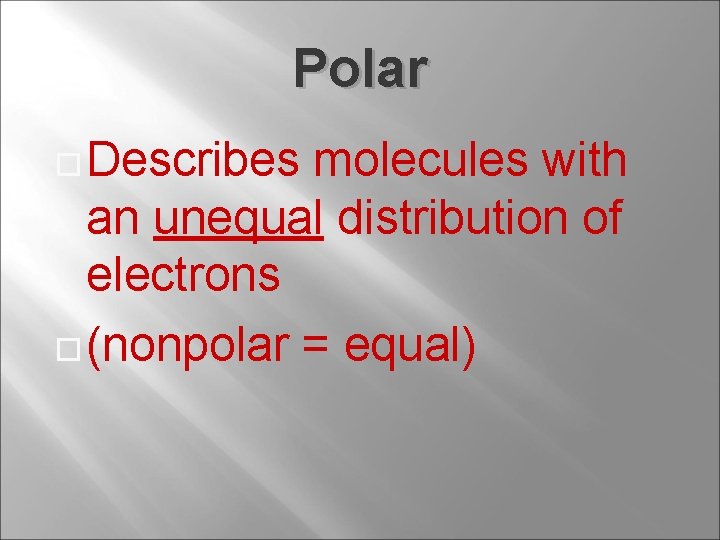
Polar Describes molecules with an unequal distribution of electrons (nonpolar = equal)

Covalent Describes a bond where electrons are shared

Ionic Describes a bond where electrons are transferred
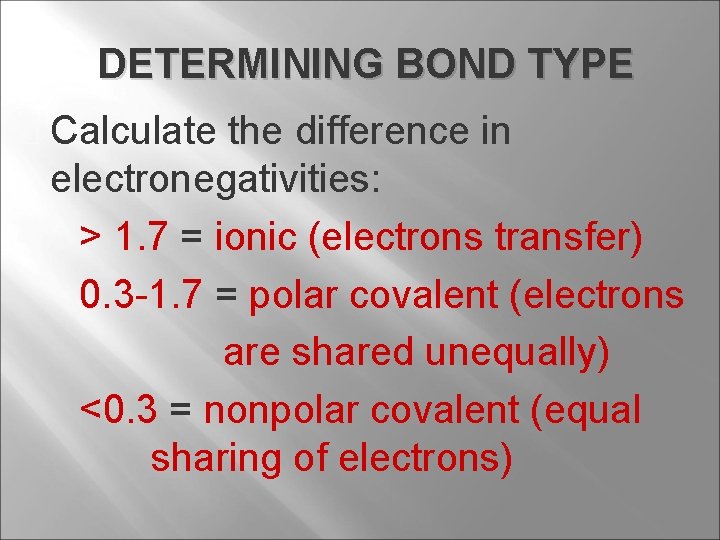
DETERMINING BOND TYPE Calculate the difference in electronegativities: > 1. 7 = ionic (electrons transfer) 0. 3 -1. 7 = polar covalent (electrons are shared unequally) <0. 3 = nonpolar covalent (equal sharing of electrons)
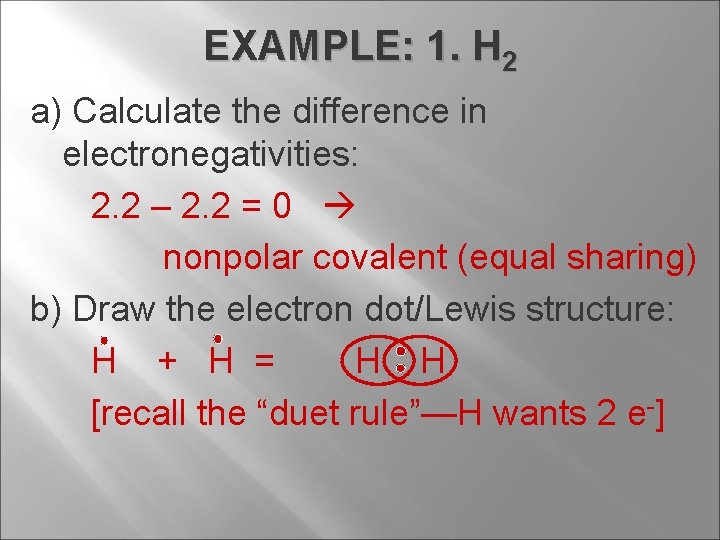
EXAMPLE: 1. H 2 a) Calculate the difference in electronegativities: 2. 2 – 2. 2 = 0 nonpolar covalent (equal sharing) b) Draw the electron dot/Lewis structure: H + H = H H [recall the “duet rule”—H wants 2 e-]
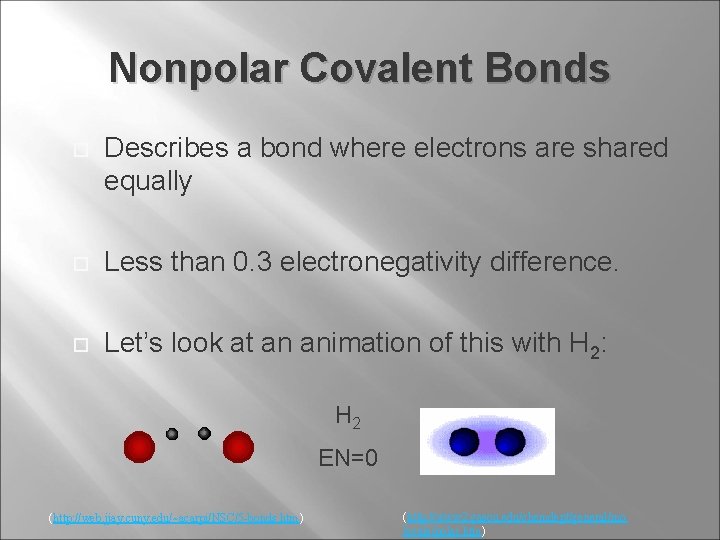
Nonpolar Covalent Bonds Describes a bond where electrons are shared equally Less than 0. 3 electronegativity difference. Let’s look at an animation of this with H 2: H 2 EN=0 (http: //web. jjay. cuny. edu/~acarpi/NSC/5 -bonds. htm) (http: //www 2. gasou. edu/chemdept/general/mo lecule/polar. htm)
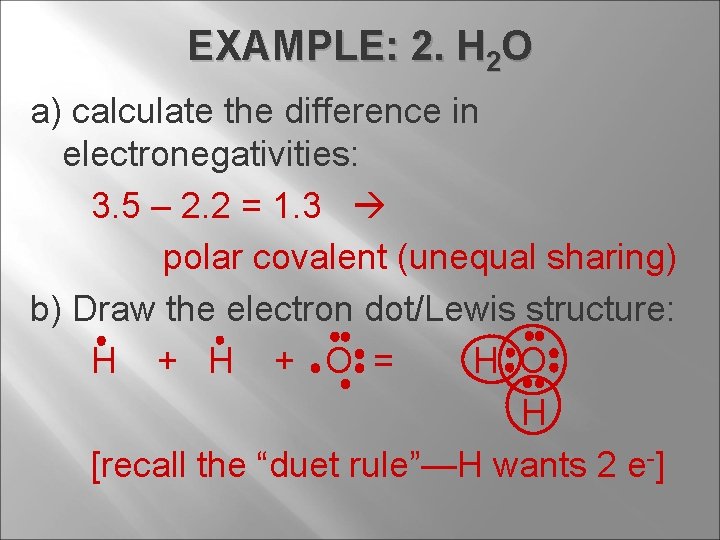
EXAMPLE: 2. H 2 O a) calculate the difference in electronegativities: 3. 5 – 2. 2 = 1. 3 polar covalent (unequal sharing) b) Draw the electron dot/Lewis structure: H + O = H O H [recall the “duet rule”—H wants 2 e-]
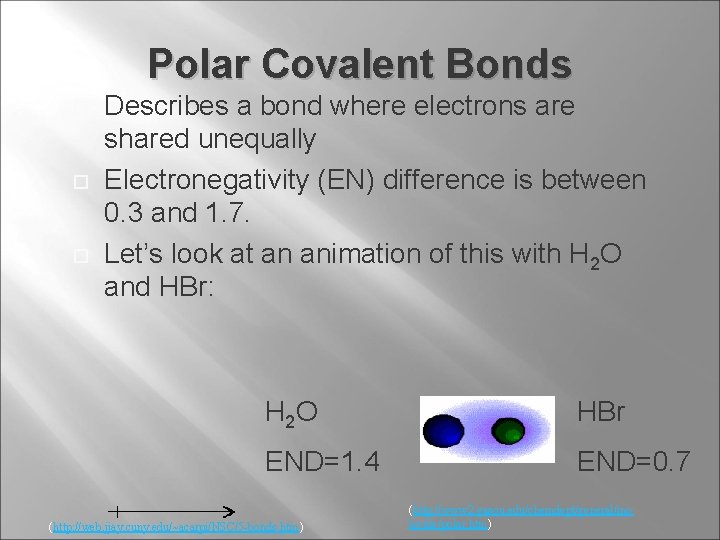
Polar Covalent Bonds Describes a bond where electrons are shared unequally Electronegativity (EN) difference is between 0. 3 and 1. 7. Let’s look at an animation of this with H 2 O and HBr: H 2 O HBr END=1. 4 END=0. 7 (http: //web. jjay. cuny. edu/~acarpi/NSC/5 -bonds. htm) (http: //www 2. gasou. edu/chemdept/general/mo lecule/polar. htm)
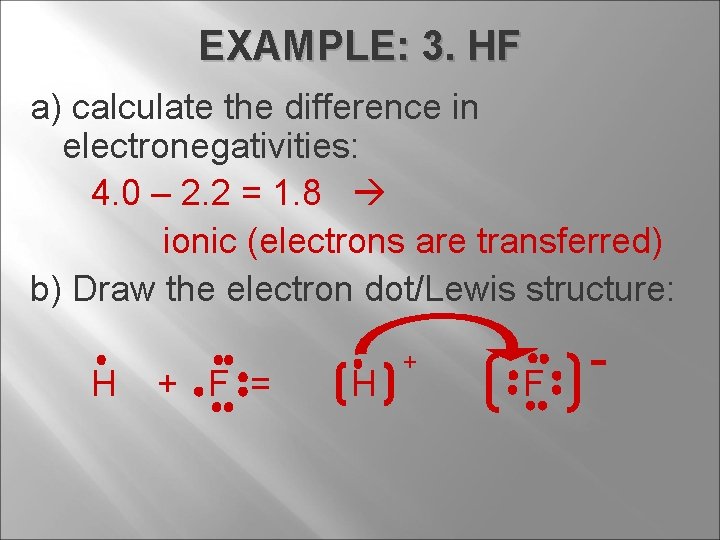
EXAMPLE: 3. HF a) calculate the difference in electronegativities: 4. 0 – 2. 2 = 1. 8 ionic (electrons are transferred) b) Draw the electron dot/Lewis structure: H + F = H + F -
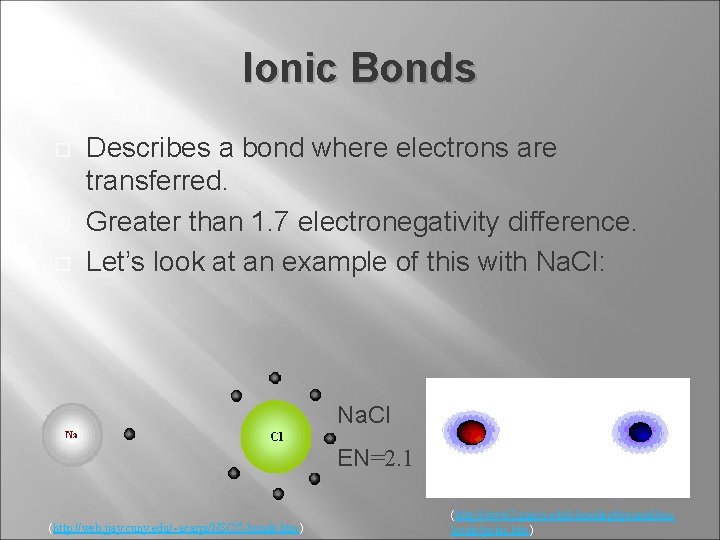
Ionic Bonds Describes a bond where electrons are transferred. Greater than 1. 7 electronegativity difference. Let’s look at an example of this with Na. Cl: Na. Cl EN=2. 1 (http: //web. jjay. cuny. edu/~acarpi/NSC/5 -bonds. htm) (http: //www 2. gasou. edu/chemdept/general/mo lecule/polar. htm)
- Slides: 13