Introduction to Balancing Equations Law of Conservation of

Introduction to Balancing Equations
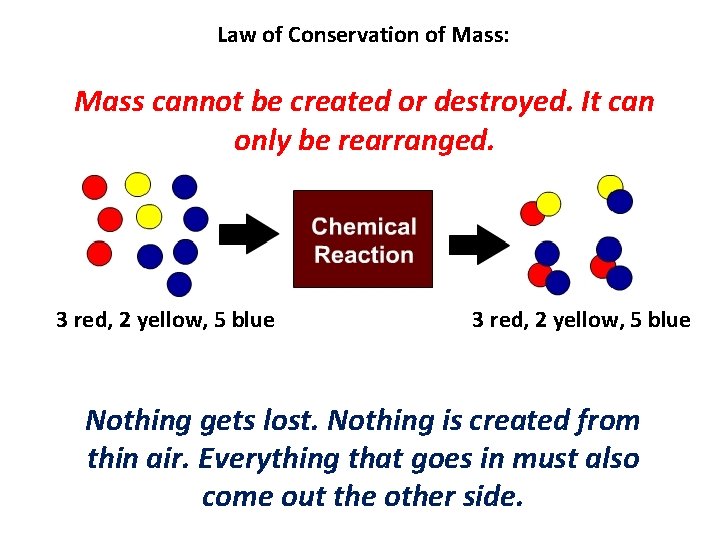
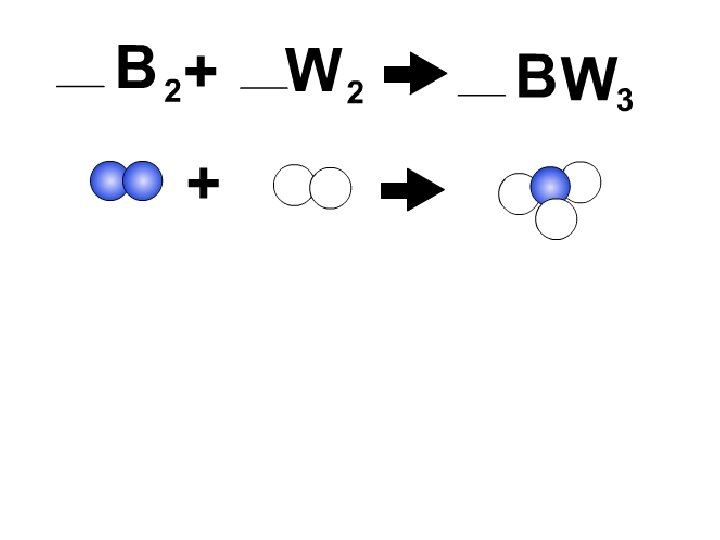
Law of Conservation of Mass: Mass cannot be created or destroyed. It can only be rearranged. 3 red, 2 yellow, 5 blue Nothing gets lost. Nothing is created from thin air. Everything that goes in must also come out the other side.
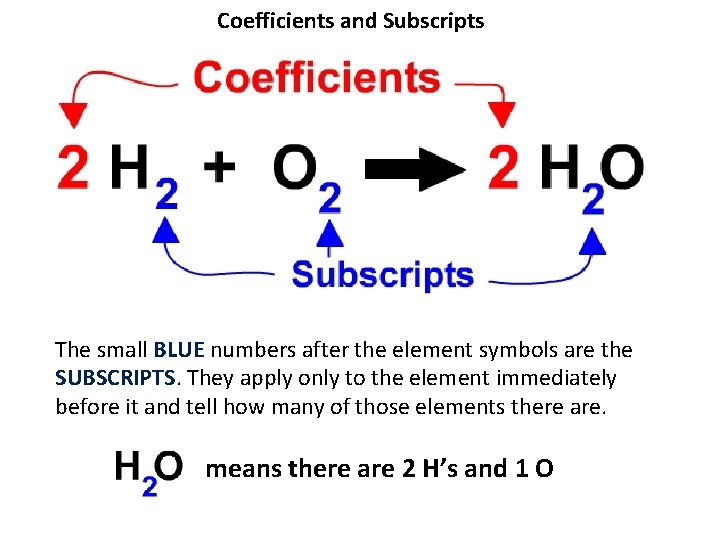
Coefficients and Subscripts The small BLUE numbers after the element symbols are the SUBSCRIPTS. They apply only to the element immediately before it and tell how many of those elements there are. means there are 2 H’s and 1 O
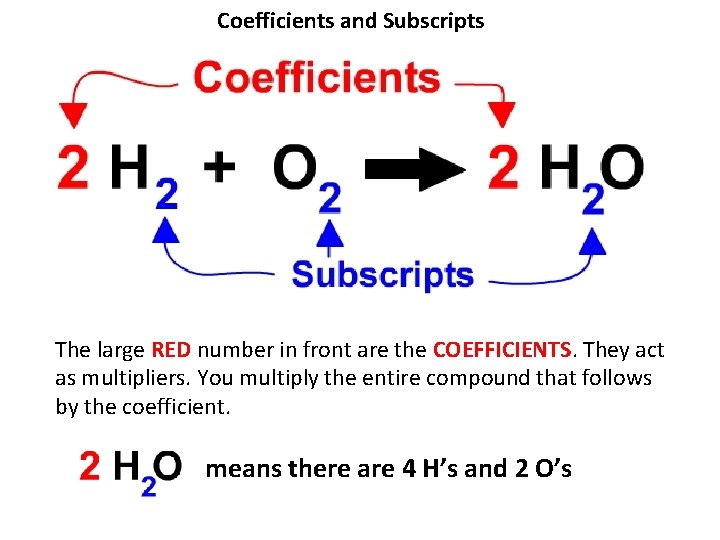
Coefficients and Subscripts The large RED number in front are the COEFFICIENTS. They act as multipliers. You multiply the entire compound that follows by the coefficient. means there are 4 H’s and 2 O’s
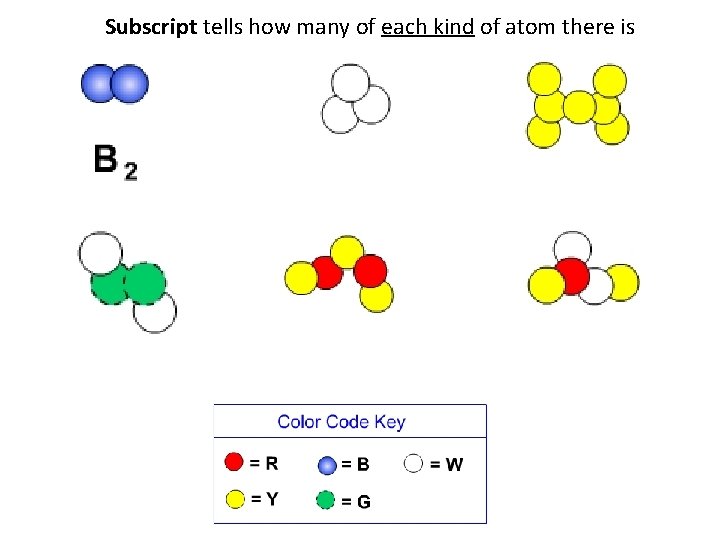
Subscript tells how many of each kind of atom there is
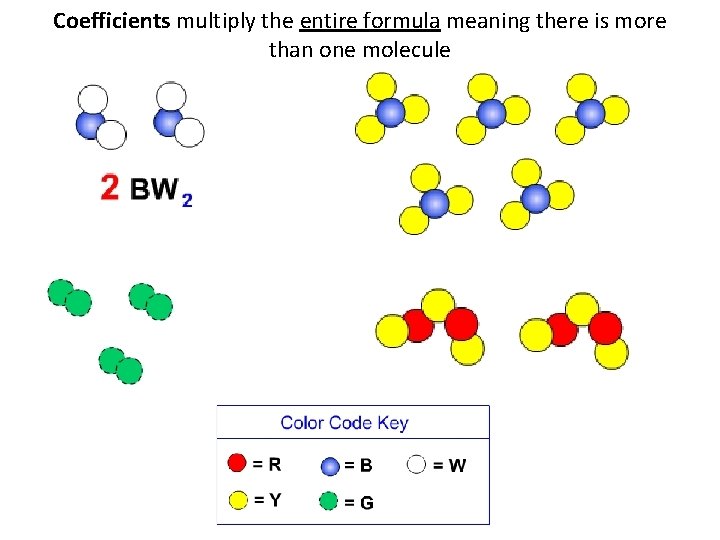
Coefficients multiply the entire formula meaning there is more than one molecule
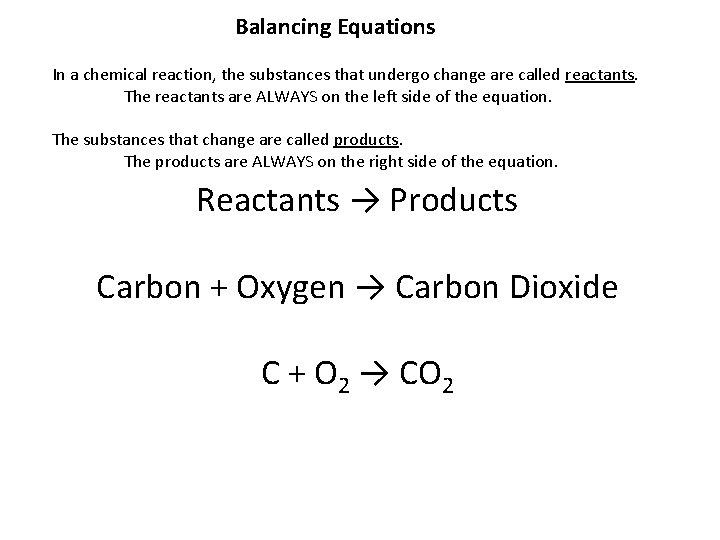
Balancing Equations In a chemical reaction, the substances that undergo change are called reactants. The reactants are ALWAYS on the left side of the equation. The substances that change are called products. The products are ALWAYS on the right side of the equation. Reactants → Products Carbon + Oxygen → Carbon Dioxide C + O 2 → CO 2
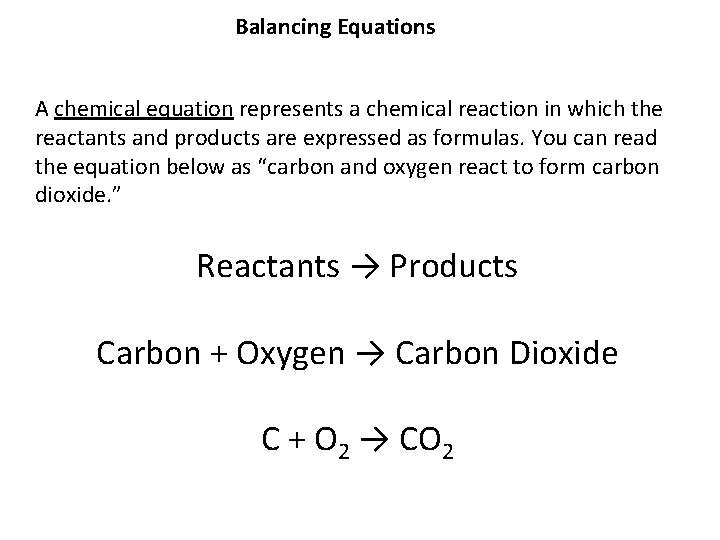
Balancing Equations A chemical equation represents a chemical reaction in which the reactants and products are expressed as formulas. You can read the equation below as “carbon and oxygen react to form carbon dioxide. ” Reactants → Products Carbon + Oxygen → Carbon Dioxide C + O 2 → CO 2
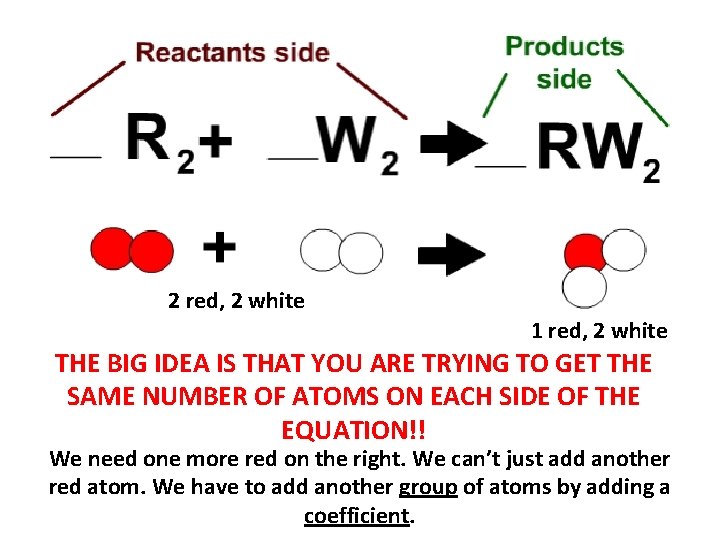
2 red, 2 white 1 red, 2 white THE BIG IDEA IS THAT YOU ARE TRYING TO GET THE SAME NUMBER OF ATOMS ON EACH SIDE OF THE EQUATION!! We need one more red on the right. We can’t just add another red atom. We have to add another group of atoms by adding a coefficient.
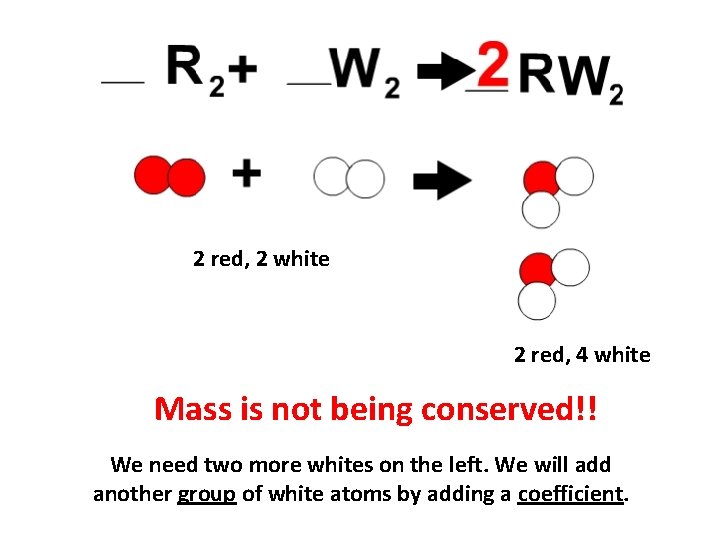
2 red, 2 white 2 red, 4 white Mass is not being conserved!! We need two more whites on the left. We will add another group of white atoms by adding a coefficient.
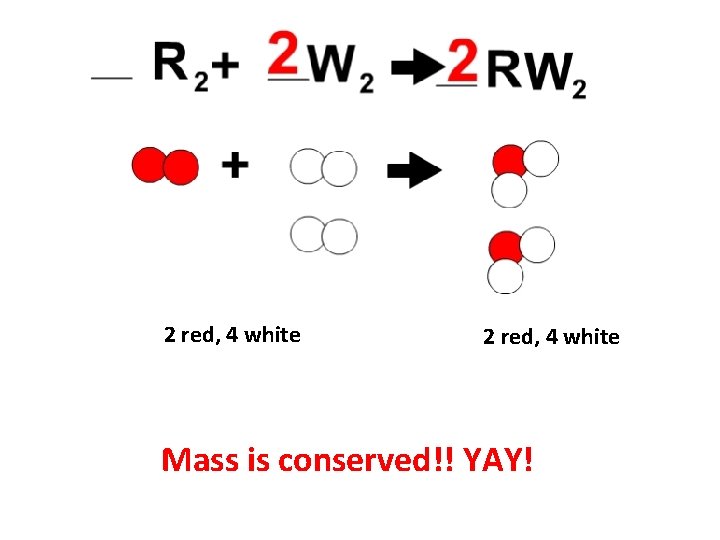
2 red, 4 white Mass is conserved!! YAY!
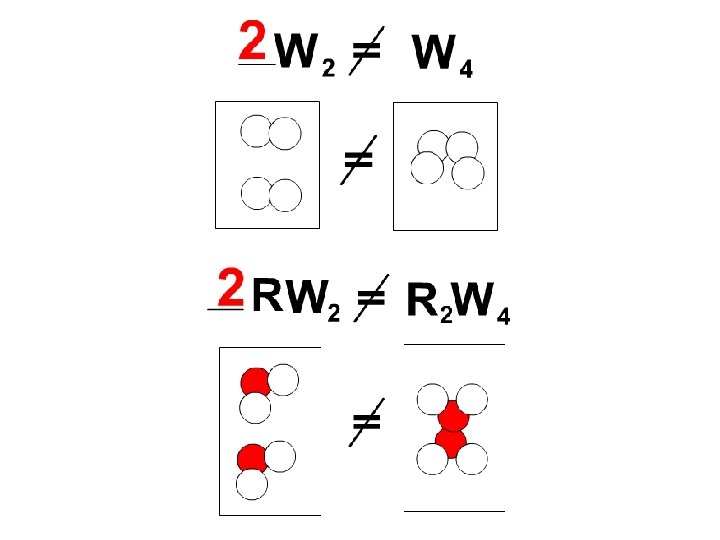
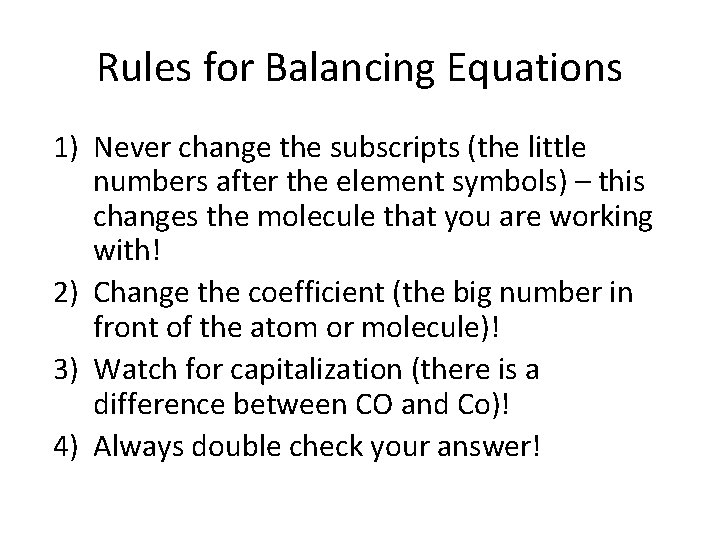
Rules for Balancing Equations 1) Never change the subscripts (the little numbers after the element symbols) – this changes the molecule that you are working with! 2) Change the coefficient (the big number in front of the atom or molecule)! 3) Watch for capitalization (there is a difference between CO and Co)! 4) Always double check your answer!
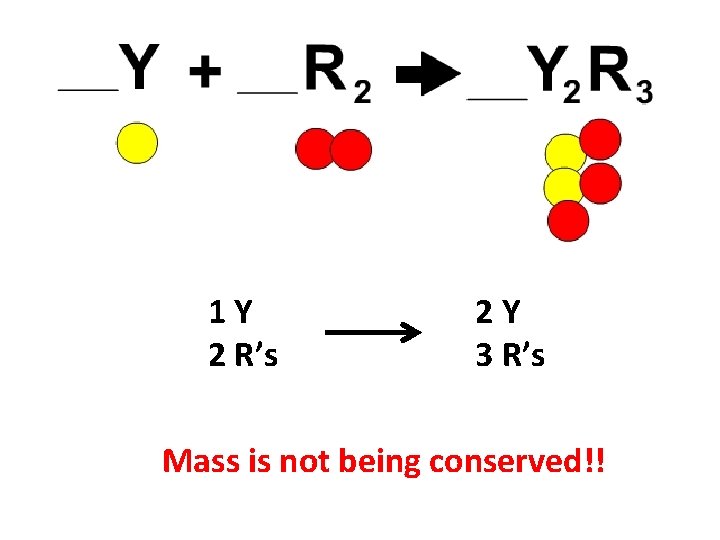
1 Y 2 R’s 2 Y 3 R’s Mass is not being conserved!!
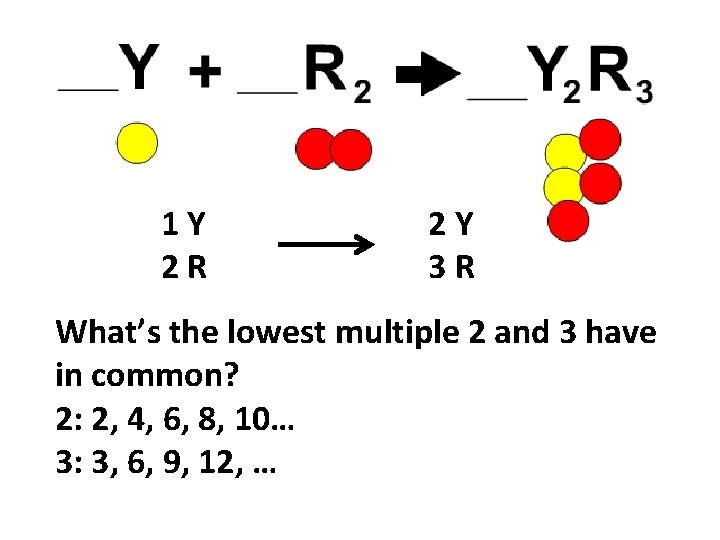
1 Y 2 R 2 Y 3 R What’s the lowest multiple 2 and 3 have in common? 2: 2, 4, 6, 8, 10… 3: 3, 6, 9, 12, …
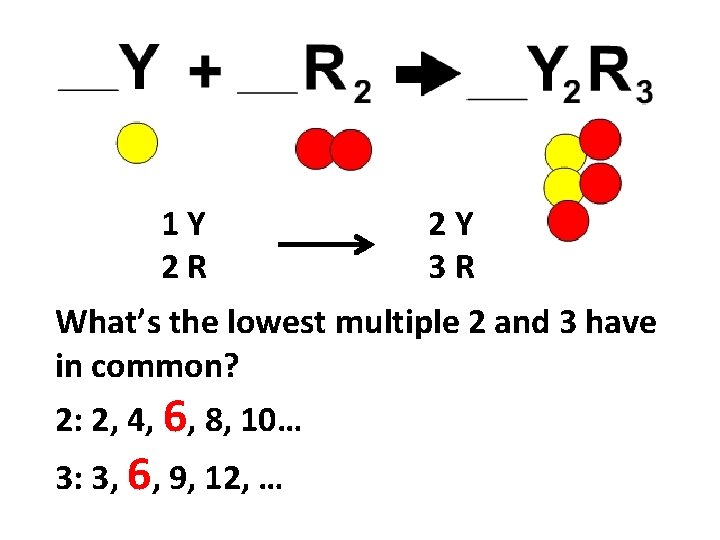
1 Y 2 R 2 Y 3 R What’s the lowest multiple 2 and 3 have in common? 2: 2, 4, 6, 8, 10… 3: 3, 6, 9, 12, …
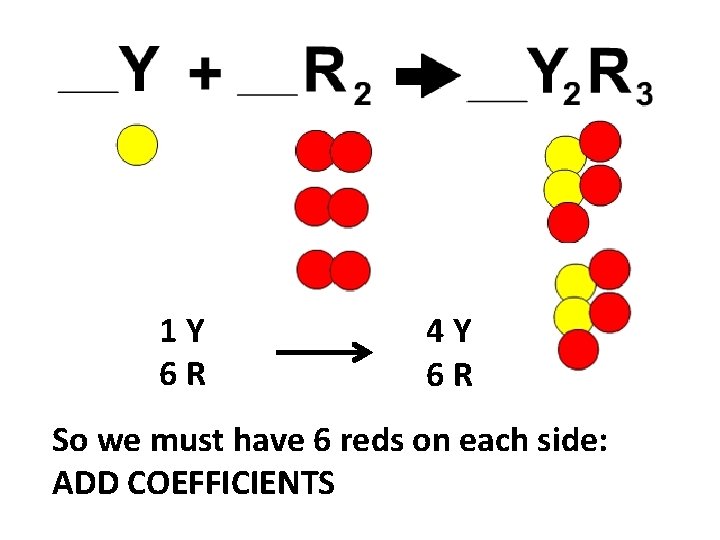
1 Y 6 R 4 Y 6 R So we must have 6 reds on each side: ADD COEFFICIENTS
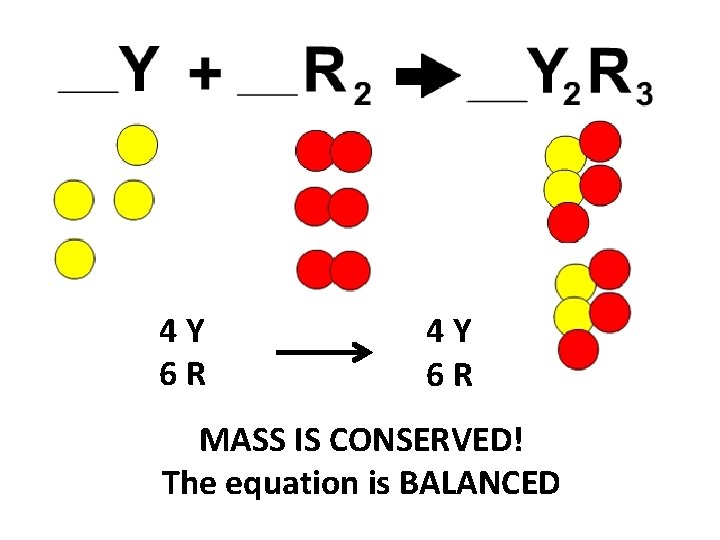
4 Y 6 R MASS IS CONSERVED! The equation is BALANCED
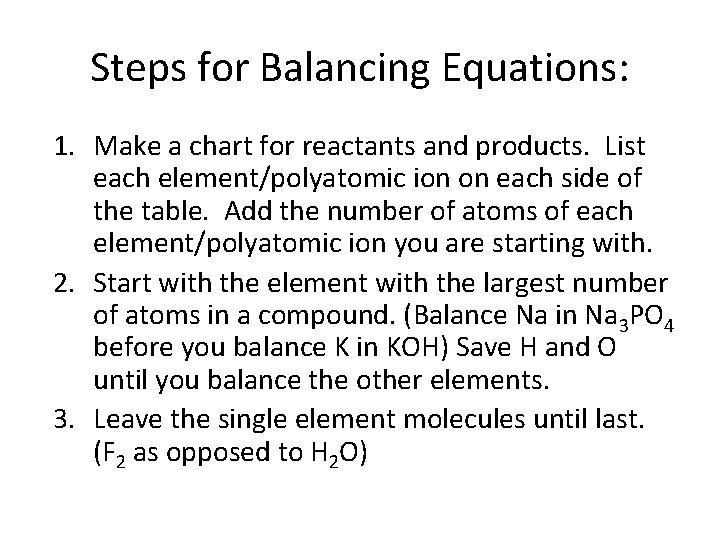
Steps for Balancing Equations: 1. Make a chart for reactants and products. List each element/polyatomic ion on each side of the table. Add the number of atoms of each element/polyatomic ion you are starting with. 2. Start with the element with the largest number of atoms in a compound. (Balance Na in Na 3 PO 4 before you balance K in KOH) Save H and O until you balance the other elements. 3. Leave the single element molecules until last. (F 2 as opposed to H 2 O)
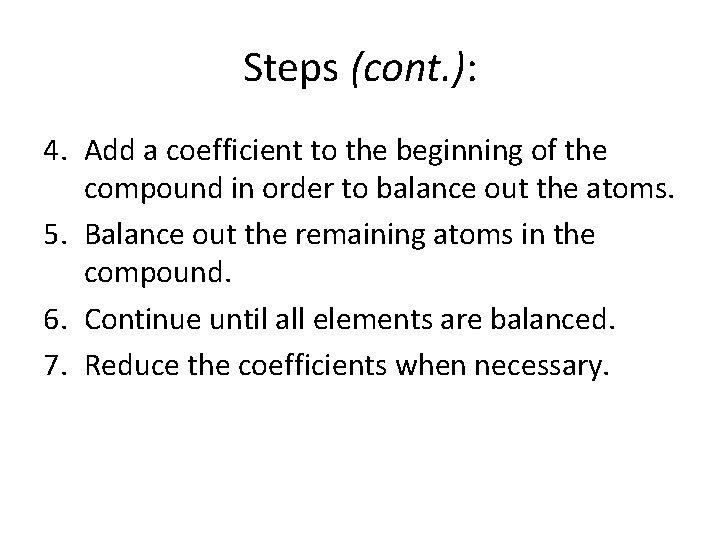
Steps (cont. ): 4. Add a coefficient to the beginning of the compound in order to balance out the atoms. 5. Balance out the remaining atoms in the compound. 6. Continue until all elements are balanced. 7. Reduce the coefficients when necessary.
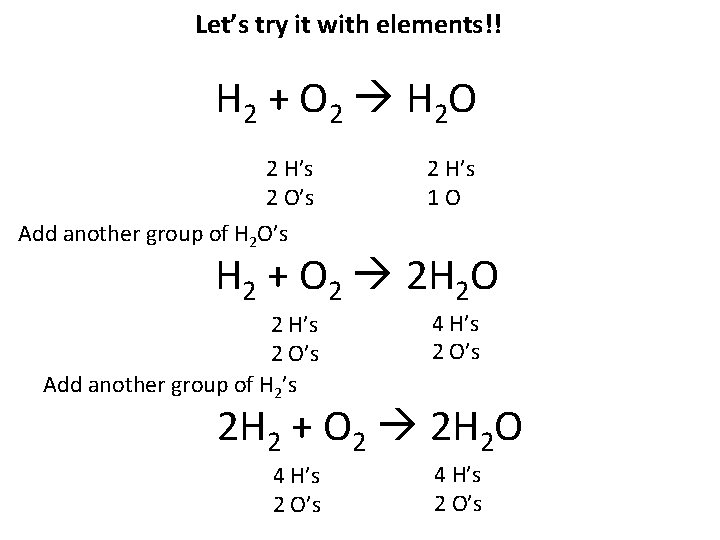
Let’s try it with elements!! H 2 + O 2 H 2 O 2 H’s 2 O’s Add another group of H 2 O’s 2 H’s 1 O 2 H’s 2 O’s Add another group of H 2’s 4 H’s 2 O’s H 2 + O 2 2 H 2 O 2 H 2 + O 2 2 H 2 O

YOU try it with elements!! Cu + O 2 Cu 2 O 1. How many Cu’s on the left? How many on the right? Balance them. 2. How many O’s on the left? How many on the right? Balance them. 3. Repeat steps 1 & 2 until the equation is balanced.
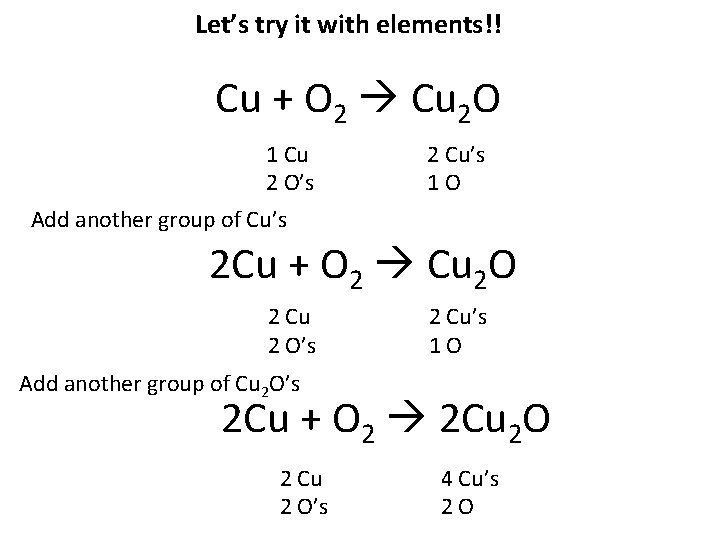
Let’s try it with elements!! Cu + O 2 Cu 2 O 1 Cu 2 O’s 2 Cu’s 1 O Add another group of Cu’s 2 Cu + O 2 Cu 2 O 2 Cu 2 O’s 2 Cu’s 1 O Add another group of Cu 2 O’s 2 Cu + O 2 2 Cu 2 O 2 Cu 2 O’s 4 Cu’s 2 O

2 Cu + O 2 2 Cu 2 O 2 Cu’s 2 O’s 4 Cu’s 2 O’s Add two more groups of Cu’s 4 Cu + O 2 2 Cu 2 O 4 Cu’s 2 O’s
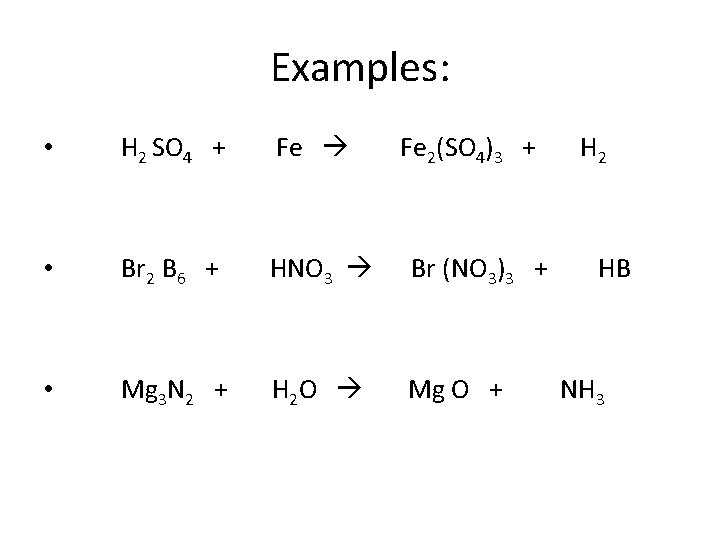
Examples: • H 2 SO 4 + Fe 2(SO 4)3 + H 2 • Br 2 B 6 + HNO 3 Br (NO 3)3 + HB • Mg 3 N 2 + H 2 O Mg O + NH 3
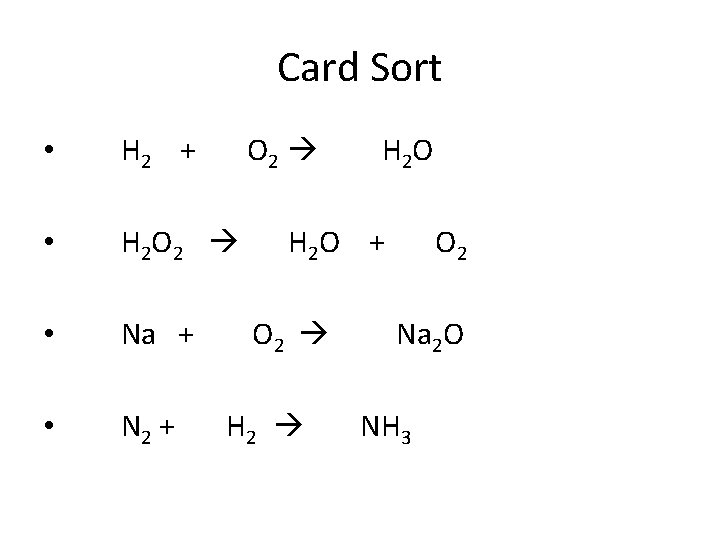
Card Sort • H 2 + O 2 H 2 O • H 2 O 2 H 2 O + O 2 • Na + O 2 Na 2 O • N 2 + H 2 NH 3
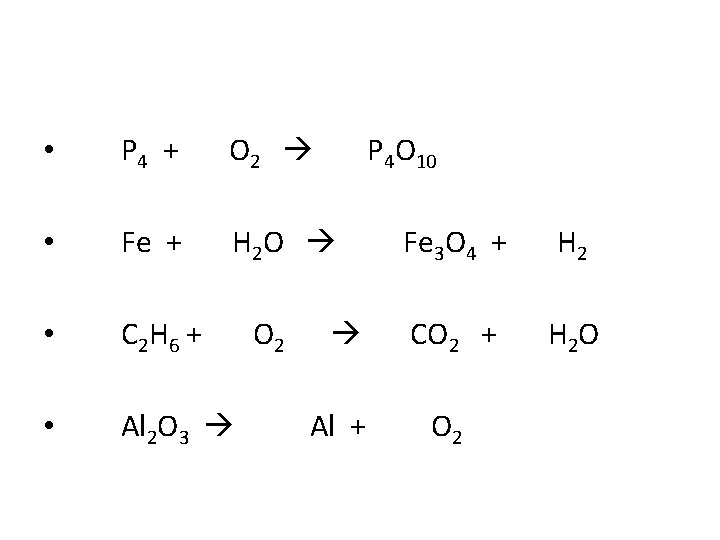
• P 4 + O 2 P 4 O 10 • Fe + H 2 O Fe 3 O 4 + H 2 • C 2 H 6 + O 2 CO 2 + H 2 O • Al 2 O 3 Al + O 2
- Slides: 28