Introduction to Atoms Ions and Isotopes What are





- Slides: 15

Introduction to Atoms, Ions and Isotopes

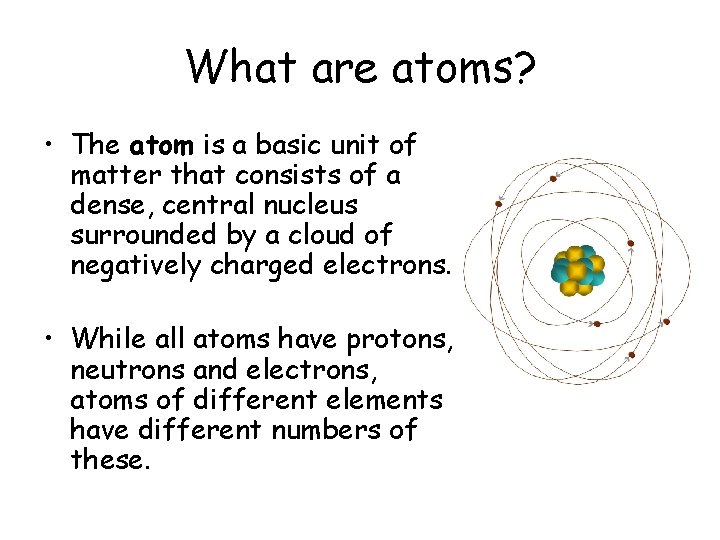
What are atoms? • The atom is a basic unit of matter that consists of a dense, central nucleus surrounded by a cloud of negatively charged electrons. • While all atoms have protons, neutrons and electrons, atoms of different elements have different numbers of these.

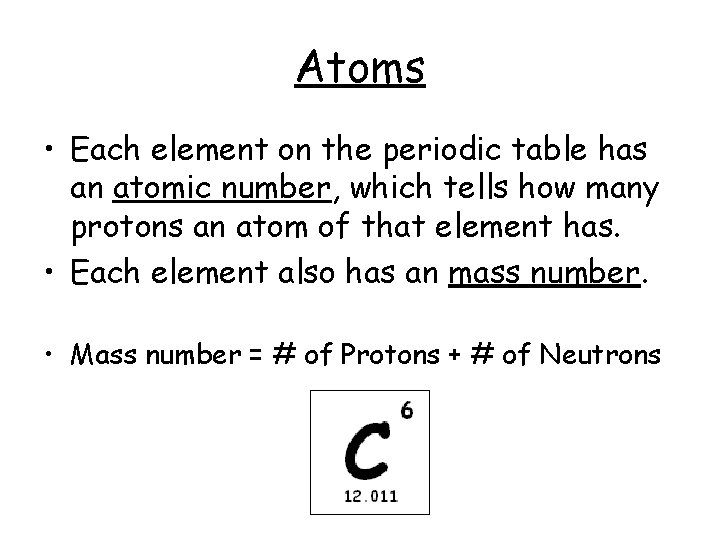
Atoms • Each element on the periodic table has an atomic number, which tells how many protons an atom of that element has. • Each element also has an mass number. • Mass number = # of Protons + # of Neutrons

Electrons • Neutral atoms have the same number of electrons (negative charge) as they do protons (positive charge). • # of electrons = atomic number • ONLY IN NEUTRAL ATOMS

Let’s Practice! • Find Boron on the periodic table. • How many protons does an atom of Boron have? • How many neutrons? – Assume the mass number is 11 • How many electrons?

Ions • An atom or small molecule with an overall positive or negative charge due to an imbalance of protons and electrons. – # of protons does not change – # of electrons changes. – Positive charge = loss of electrons – Negative charge = gain of electron

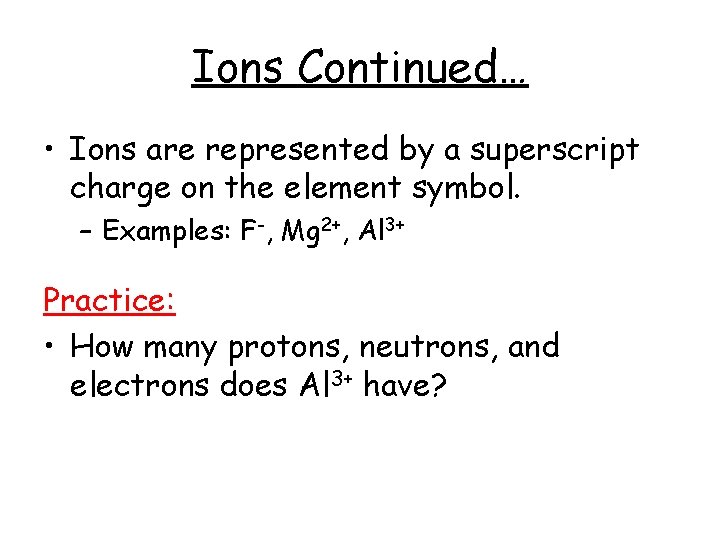
Ions Continued… • Ions are represented by a superscript charge on the element symbol. – Examples: F-, Mg 2+, Al 3+ Practice: • How many protons, neutrons, and electrons does Al 3+ have?

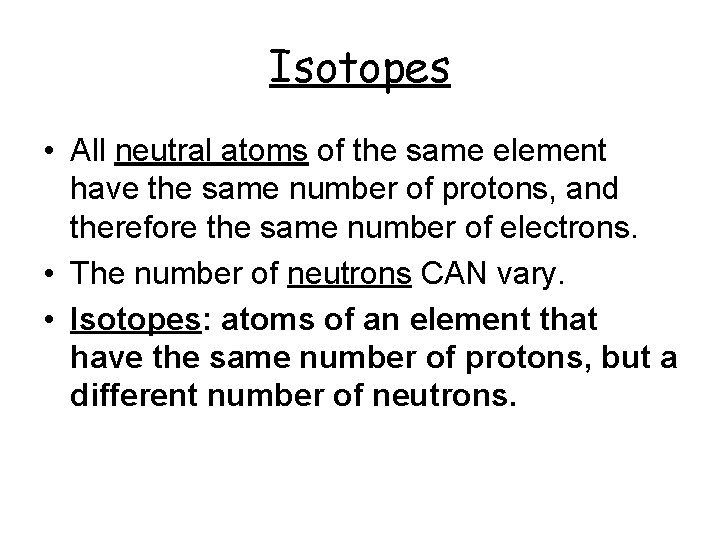
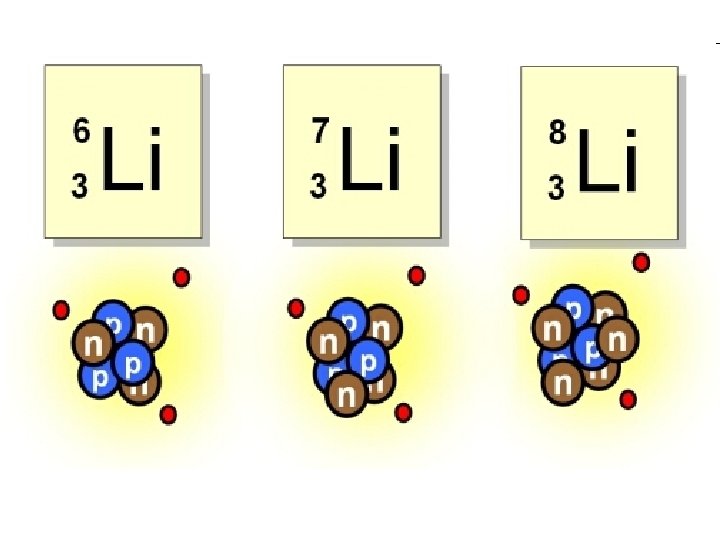
Isotopes • All neutral atoms of the same element have the same number of protons, and therefore the same number of electrons. • The number of neutrons CAN vary. • Isotopes: atoms of an element that have the same number of protons, but a different number of neutrons.


Average Atomic Mass • The mass shown on your periodic table as a decimal is the average atomic mass. – Average mass of all isotopes of an atom in nature.

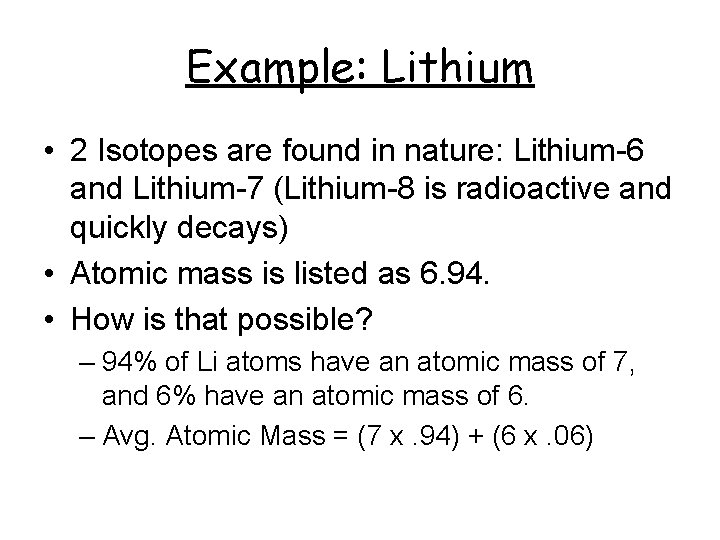
Example: Lithium • 2 Isotopes are found in nature: Lithium-6 and Lithium-7 (Lithium-8 is radioactive and quickly decays) • Atomic mass is listed as 6. 94. • How is that possible? – 94% of Li atoms have an atomic mass of 7, and 6% have an atomic mass of 6. – Avg. Atomic Mass = (7 x. 94) + (6 x. 06)

Complete Atomic Structure WS #1 Front and Back Hint: Chromium-52 means Chromium with a mass number of 52. 26 Fe means iron with atomic number 26.

Let’s Review! • ____ are charged atoms. – _____ ions have LOST electrons. – _____ ions have GAINED electrons. • An atom of Cu 2+ with mass number 63 has: – ____ protons – ____ neutrons – ____ electrons

More Review • ______ are atoms of the same element that have different numbers of neutrons in the nucleus. • What does average atomic mass represent?

Related Book Work • Read section 5. 1 • Pg. 134 -143 • Answer the following questions on page 162 1 -3, 6, 7, 29, 31, 32, 38, 65, 71 -76.