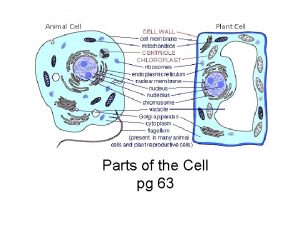
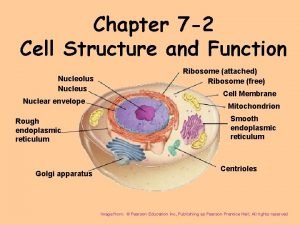
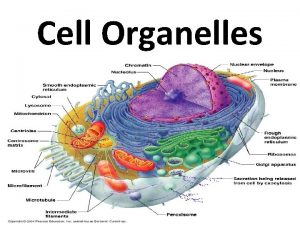
Introduction to Animal Cell Culture Introduction Cell culture






























- Slides: 44

Introduction to Animal Cell Culture

Introduction � Cell culture is the process by which prokaryotic, eukaryotic or plant cells are grown under controlled conditions. But in practice it refers to the culturing of cells derived from animal cells. � Cell culture was first successfully undertaken by Ross Harrison, 1907 & Carrel, 1912. � Roux in 1885 for the first time maintained embryonic chick cells in a cell culture.

Historical events in the development of cell culture � 1878: Claude Bernard proposed that physiological systems of an organism can be maintained in a living system after the death of an organism. � 1885: Roux maintained embryonic chick cells in a saline culture. � 1897: Loeb demonstrated the survival of cells isolated from blood and connective tissue in serum and plasma.

� 1903: Jolly observed cell division of salamander leucocytes in vitro. � 1907: Harrison cultivated frog nerve cells in a lymph clot held by the 'hanging drop' method and observed the growth of nerve fibers in vitro for several weeks. (father of cell culture). � 1910: Burrows succeeded in long term cultivation of chicken embryo cell in plasma clots.

� 1911: Lewis and Lewis made the first liquid media consisted of sea water, serum, embryo extract, salts and peptones. They observed limited monolayer growth. � 1916: Rous and Jones introduced proteolytic enzyme trypsin for the subculture of adherent cells. � 1927: Carrel and Rivera produced the first viral vaccine - Vaccinia.

� 1940 s: The use of the antibiotics penicillin and streptomycin in culture medium decreased the problem of contamination in cell culture. � 1948: Earle isolated mouse L fibroblasts which formed clones from single cells. Fischer developed a chemically defined medium, CMRL 1066. � 1952: Gey established a continuous cell line from a human cervical carcinoma known as He. La (Helen Lane) cells.

� 1955: Eagle studied the nutrient requirements of selected cells in culture and established the first widely used chemically defined medium. (EMEM) � 1964: Littlefield introduced the HAT medium for cell selection. � 1965: Harris and Watkins were able to fuse human and mouse cells by the use of a virus. � 1975: Kohler and Milstein produced the first hybridoma capable of secreting a monoclonal antibody.

1982: Human insulin became the first recombinant protein to be licensed as a therapeutic agent. � � 1986: Lymphoblastoid γ-IFN licensed. 1987: Tissue-type plasminogen activator from recombinant animal cells became commercially available. � � 1989: Recombinant erythropoietin in trial. 1990: Recombinant products in clinical trial (factor VIII, HIVgp 120, CD 4, GM-CSF, EGF, m. Abs, IL-2). �

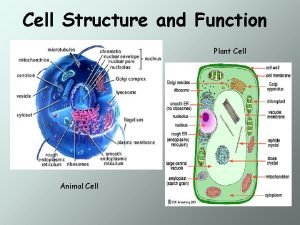
Animal Cell Characteristics 10 -30 um larger than bacteria or yeast � Eukaryotic � Cell membrane – no cell wall: shear sensitivity � Surface is negatively charged – grow on positively charged surfaces � � Suspension cells or anchorage dependant cells. � 80 -85% water, 10 -20% protein, and 1 -5% carbohydrates. � Lipid bilayer cell membrane that is sensitive to shear. � Optimum growth at 37 o. C

Tissue culture In vitro cultivation of organs, tissues & cells at defined temperature using an incubator & supplemented with a medium containing cell nutrients & growth factors is collectively known as tissue culture. � Different types of cell grown in culture includes connective tissue elements such as fibroblasts, skeletal tissue, cardiac, epithelial tissue (liver, breast, skin, kidney) and many different types of tumor cells. �

Common theme to all cell cultures Cell density must be controlled & optimized � Want cell density to be high enough to maximize production of (usually) protein product � But if cell density gets too high: › Nutrients in media get depleted › Waste products build up in media › In the case of some cells (such as animal cells), cell-to-cell contact can cause cells to stop dividing (“contact inhibition”) �

Why is cell culture used for? Areas where cell culture technology is currently playing a major role� Model systems for: - Studying basic cell biology - Interactions between disease causing agents and cells - Effects of drugs on cells - Process and triggering of aging & nutritional studies

Toxicity testing: - Study the effects of new drugs � Cancer research: - Study the function of various chemicals, virus & radiation to convert normal cultured cells to cancerous cells �

Culturing of cells Cells are cultured as anchorage dependent or independent � Cell lines derived from normal tissues are considered as anchorage-dependent grows only on a suitable substrate e. g. tissue cells � Suspension cells are anchorage-independent e. g. blood cells � Transformed cell lines either grows as monolayer or as suspension �

Cells are either…. � Anchorage – dependant � Anchorage - independant

Anchorage – dependant cells � Most animal derived cells � Adhere to bottom of a flask and form a monolayer � Eventually cover entire surface of substratum � Proliferation then stops � Need to subculture cells at this point (remove to fresh medium) � Proliferation can begin again

Anchorage – independant cells � Cells associated with body fluid -blood cells � Grown in suspension � Will eventually need subculturing

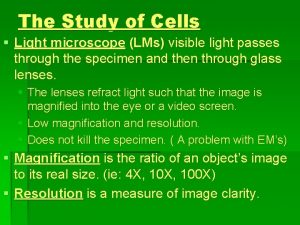
Basic equipments used in cell culture Laminar cabinet-Vertical are preferable � Incubation facilities- Temperature of 25 -30 o. C for insect & 37 o. C for mammalian cells, 95% air at 99% relative humidity. To prevent cell death incubators set to cut out at approx. 38. 5 o. C � Refrigerators- Liquid media kept at 4 o. C , enzymes (e. g. trypsin) & media components (e. g. glutamine & serum) at -20 o. C � Microscope- An inverted microscope with 10 x to 100 x magnification � Tissue culture ware- Culture plastic ware treated by polystyrene �

Basic aseptic conditions � If working on the bench use a Bunsen flame to heat the air surrounding the Bunsen. � Swab all bottle tops & necks with 70% ethanol. � Flame all bottle necks & pipette by passing very quickly through the hottest part of the flame.

Avoiding placing caps & pipettes down on the bench; practice holding bottle tops with the little finger � Work either left to right or vice versa, so that all material goes to one side, once finished � Clean up spills immediately & always leave the work place neat & tidy. �

Safety aspect in cell culture Possibly keep cultures free of antibiotics in order to be able to recognize the contamination � Never use the same media bottle for different cell lines. If caps are dropped or bottles touched unconditionally touched, replace them with new ones � Necks of glass bottles prefer heat at least for 60 seconds at a temperature of 200 o. C � Switch on the laminar flow cabinet 20 min. prior to start working � Cell cultures which are frequently used should be subcultered & stored as duplicate strains �

Medium Glucose energy source up 55 mmol/L � Glutamine energy source 2 -7 mmol/L � Aerobically metabolize to CO 2 or anaerobically to lactic acid. � Lactate and ammonium toxic byproducts of mammalian cell growth. � Lactic acid and ammonium inhibitory at 30 and 5 mmol/L, respectively. � Lactate reduces p. H. �

Oxygen utilized at approximately 0. 05 -5 pmol O 2/cell 10 -30% DO is non-limiting. Higher DO concentrations can be toxic, leading to oxidative damage. � Amino acids: cell line dependant, balance is critical. � Growth factors � Cytokines � Trace elements �

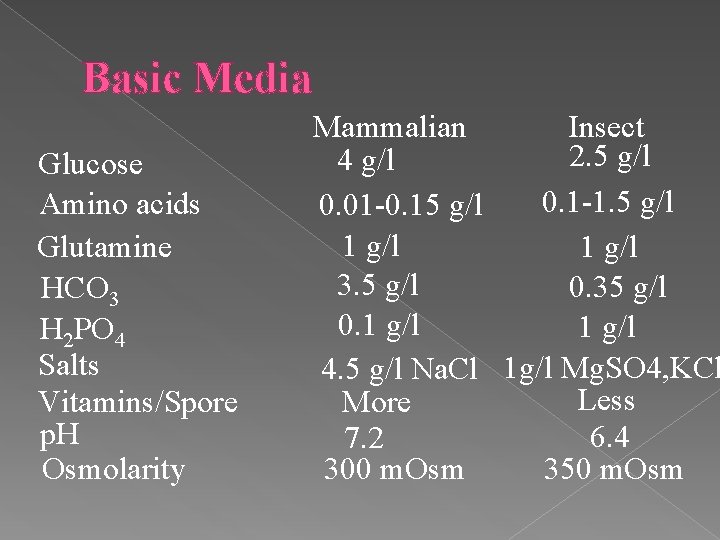
Basic Media Glucose Amino acids Glutamine HCO 3 H 2 PO 4 Salts Vitamins/Spore p. H Osmolarity Mammalian Insect 2. 5 g/l 4 g/l 0. 1 -1. 5 g/l 0. 01 -0. 15 g/l 1 g/l 3. 5 g/l 0. 35 g/l 0. 1 g/l 4. 5 g/l Na. Cl 1 g/l Mg. SO 4, KCl Less More 6. 4 7. 2 300 m. Osm 350 m. Osm

Serum: The clear liquid that separates from the blood when it is allowed to clot. Fetal Bovine Serum (FBS; also named as 'FCS') � widely used in animal cell culture as an essential supplement. � serum and protein free media have only been established for selected protocols. � by-product of the beef-packing industry, FBS can only be obtained where sufficient numbers of fetuses become available during the slaughtering process. �

Serum Component - Albumin - Immunoglobulins (Ig. G 75 -85% of all Ig) - Fibrinogen - Alpha-1 antitrypsin - Alpha-2 macroglobulin - Transferrin - Alpha-2+ß-lipoproteins (LDL) - Alpha-lipoproteins (HDL) - Haptoglobin - Alpha-1 acid glycoprotein

� • • • Ions Bicarbonate 25 -35 m. M Chloride 100 -108 m. M Sodium 134 -143 m. M Potassium 3. 5 -4. 5 m. M Calcium 2 -2. 5 m. M p. H 7. 4

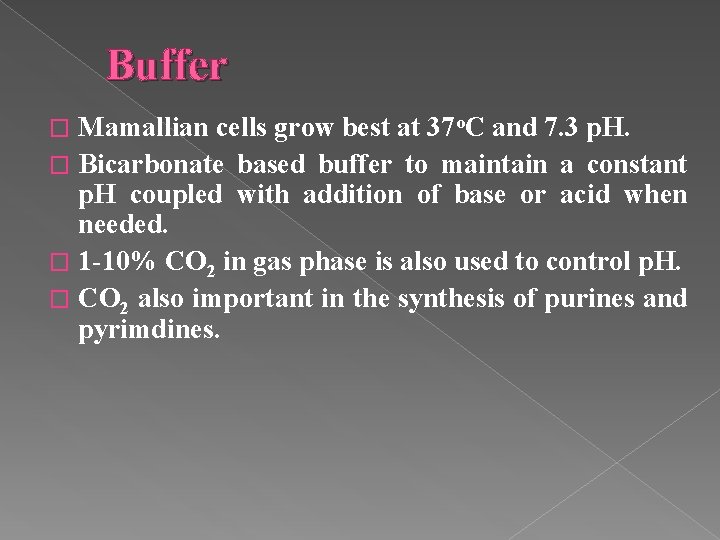
Buffer Mamallian cells grow best at 37 o. C and 7. 3 p. H. � Bicarbonate based buffer to maintain a constant p. H coupled with addition of base or acid when needed. � 1 -10% CO 2 in gas phase is also used to control p. H. � CO 2 also important in the synthesis of purines and pyrimdines. �

CO 2 primes energy metabolism. � Excess CO 2 suppresses cell growth and can alter intracellular p. H. � Osmolarity increases as p. H is adjusted due to the addition of salts, too high osmolarity results in cell shrinkage and eventually lysis. �

Tissue Culture Applications Cell Products � Cell-Cell Interaction � Intracellular Activity � Intracellular Flux � Environmental Interaction �

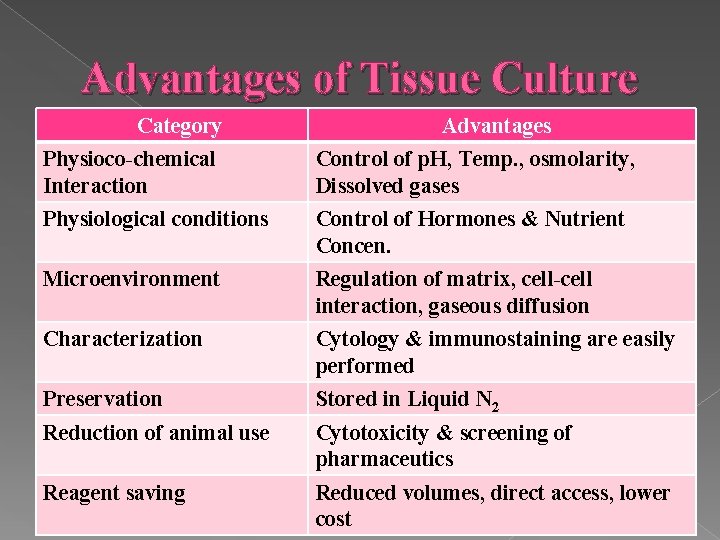
Advantages of Tissue Culture Category Advantages Physioco-chemical Interaction Control of p. H, Temp. , osmolarity, Dissolved gases Physiological conditions Control of Hormones & Nutrient Concen. Microenvironment Regulation of matrix, cell-cell interaction, gaseous diffusion Characterization Cytology & immunostaining are easily performed Preservation Stored in Liquid N 2 Reduction of animal use Cytotoxicity & screening of pharmaceutics Reagent saving Reduced volumes, direct access, lower cost

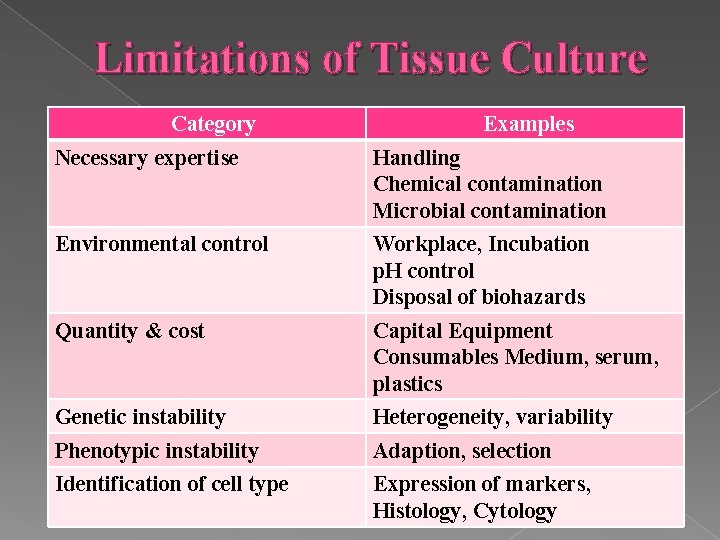
Limitations of Tissue Culture Category Examples Necessary expertise Handling Chemical contamination Microbial contamination Environmental control Workplace, Incubation p. H control Disposal of biohazards Quantity & cost Capital Equipment Consumables Medium, serum, plastics Genetic instability Heterogeneity, variability Phenotypic instability Adaption, selection Identification of cell type Expression of markers, Histology, Cytology

Primary culture Cells when surgically or enzymatically removed from an organism and placed in suitable culture environment will attach and grow are called as primary culture � Primary cells have a finite life span � Primary culture contains a very heterogeneous population of cells � Sub culturing of primary cells leads to the generation of cell lines �

Cell lines have limited life span, they passage several times before they become senescent � Cells such as macrophages and neurons do not divide in vitro so can be used as primary cultures � Lineage of cells originating from the primary culture is called a cell strain �

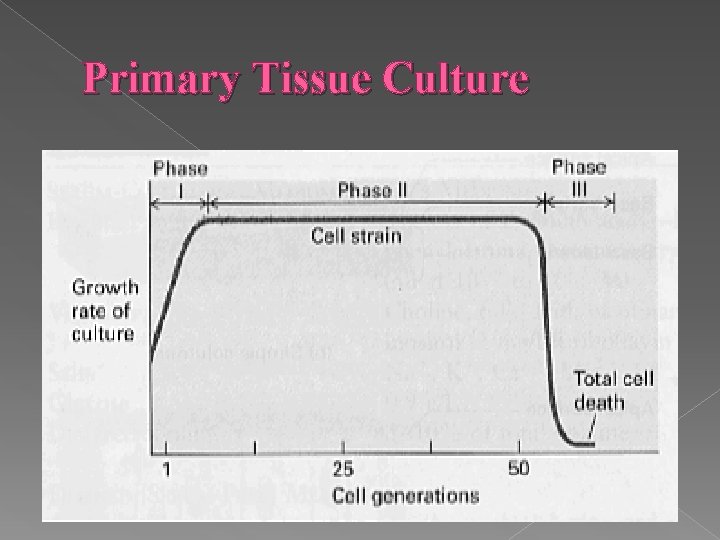
Primary Tissue Culture A culture derived directly from a tissue � A stage from cell isolation to first subculturing • Best resembling natural tissue • Limited growth potential • Limited life span • May give rise to a cell strain or be immortalized • Strain – a lineage of cells originating from one primary culture. �

Primary Tissue Culture

Tissue culture cell lines � Cell line: a permanently established cell culture that will proliferate indefinitely given appropriate fresh medium and space. › Unlike primary cells (directly from tissue), cell lines have been immortalized › Immortalization is a process that shares similarities with the transformation of a normal cell into a cancerous cell

Cell Lines Primary culture: cell recently excised from specific organs of animals. � Secondary culture: cell line obtained from the primary culture. Can be adapted to grow in suspension and are non-anchorage dependant. Will only grow for about 30 generations. � Continuous, immortal, transformed cell lines: cells that can be propagated indefinitely (cancer cell lines are all continuous). �

Types of cell line Different types of cell line produced from different tissues or organs. Broadly they are grouped into: - Finite cell line - Continuous cell line.

Finite cell line: Grow through a limited number of cell generations and have limited life. Grow slowly and form monolayer. Doubling time ranges from 24 to 96 hours. Anchorage dependent, contact inhibition and density limitation.

Continuous cell lines: Obtained from either from transformed cell line in vitro or cancerous cells. Divides rapidly. Generation time 12 -24 hrs. No or reduced density limitation

Continuous cell lines Most cell lines grow for a limited number of generations after which they ceases. � Cell lines which either occur spontaneously or induced virally or chemically transformed into Continous cell lines. �

Characteristics of continous cell lines Smaller, more rounded, less adherent with a higher nucleus /cytoplasm ratio. � Fast growth and have aneuploid chromosome number. � Reduced serum and anchorage dependence and grow more in suspension conditions. -ability to grow upto higher cell density -different in phenotypes from donor tissue -stop expressing tissue specific genes �

Mammalian cell line animal cell line. � Insect, fish, crustacean cell lines are evolving technologies. � Baculovirus: virus that infects insect cells. Nonpathogenic to humans, has a strong promoter. � Insect cell lines are naturally continuous. � Most cell lines derived from ovaries or embryonic tissue. � Hybridoma cells: fusing lymphocytes (normal blood cells that make antibodies) with myeloma (cancer) cells. �
 Bioreactor considerations for animal cell culture
Bioreactor considerations for animal cell culture Hira abbas
Hira abbas Cell city worksheet
Cell city worksheet Venn diagram plant and animal cell
Venn diagram plant and animal cell Venn diagram plant vs animal cells
Venn diagram plant vs animal cells Tonoplast
Tonoplast Cytoplasm function in plant cell
Cytoplasm function in plant cell Cell city introduction
Cell city introduction Carbohydrate side chain
Carbohydrate side chain Idealized animal cell and plant cell
Idealized animal cell and plant cell What is the gooey liquid in plant and animal cells
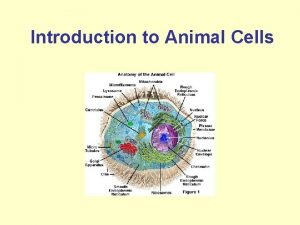
What is the gooey liquid in plant and animal cells Introduction of animal cell
Introduction of animal cell Animal rights vs animal welfare
Animal rights vs animal welfare Whats the difference between animal and plant cells
Whats the difference between animal and plant cells Difference between plant cell and bacterial cell
Difference between plant cell and bacterial cell Plant cell vacuole
Plant cell vacuole Magnification of cell
Magnification of cell Cilia on animal cell
Cilia on animal cell Plant cell structure under electron microscope
Plant cell structure under electron microscope Animal cell under microscope labeled
Animal cell under microscope labeled Cell membrane joke
Cell membrane joke Characteristics of the animal cell
Characteristics of the animal cell Cell acrostic poem
Cell acrostic poem Wolf life cycle
Wolf life cycle Composite animal cell
Composite animal cell Plant and animal cells foldable
Plant and animal cells foldable Golgi vesicles function in animal cell
Golgi vesicles function in animal cell Plant animal cell
Plant animal cell Animal cell analogy ideas
Animal cell analogy ideas Plant or animal cell
Plant or animal cell Smooth endoplasmic reticulum nickname
Smooth endoplasmic reticulum nickname How does an animal cell get energy
How does an animal cell get energy Animal cell structure grade 10
Animal cell structure grade 10 Dot
Dot Nucleolus gif
Nucleolus gif How does an animal cell get energy
How does an animal cell get energy What are cells made up of
What are cells made up of Garbage collector of the cell
Garbage collector of the cell How does an animal cell get energy
How does an animal cell get energy How does an animal cell get energy
How does an animal cell get energy Plant cell diagram labeled 6th grade
Plant cell diagram labeled 6th grade Corn plant
Corn plant Interphase animal cell
Interphase animal cell Animal cell
Animal cell Animal cell vacuole
Animal cell vacuole