Introduction to Analytical Chemistry CHAPTER 22 SELECTED METHODS




- Slides: 10

Introduction to Analytical Chemistry CHAPTER 22 SELECTED METHODS OF ANALYSIS

Selected Methods of Analysis This chapter contains detailed directions for performing a variety of chemical analyses. The methods have been chosen to introduce you to analytical techniques that are widely used by chemists. Your chances of success in the laboratory will greatly improve if you take time before beginning any analysis to read carefully and understand each step in the method and to develop a plan for how and when you will perform each step. 22 -2 Copyright © 2011 Cengage Learning

Background Information Before you start an analysis, you should understand the significance of each step in the procedure to avoid the pitfalls and potential sources of error. 22 -3 Copyright © 2011 Cengage Learning

The Accuracy of Measurements Generally, measurements that appear in the equation used to compute the results must be performed with maximum precision. The remaining measurements can and should be made less carefully to conserve time. 22 -4 Copyright © 2011 Cengage Learning

Water Some laboratories use deionizers to purify water; others employ stills for this purpose. The terms “distilled water” and “deionized water” are used interchangeably in the directions that follow. 22 -5 Copyright © 2011 Cengage Learning

22 A-3 Delivering an Aliquot Whenever a buret or pipet is used to deliver a measured volume of solution, the liquid it contains before measurement should have the same composition as the solution to be dispensed. 22 -6 Copyright © 2011 Cengage Learning

22 A-7 Sampling In most analytical methods, only a small fraction of the entire population is analyzed. A laboratory sample taken from the entire batch must be representative of the population. If p represents the fraction of the particles of the analyte, then 1 – p is the fraction of the second type of particles. 22 -7 Copyright © 2011 Cengage Learning

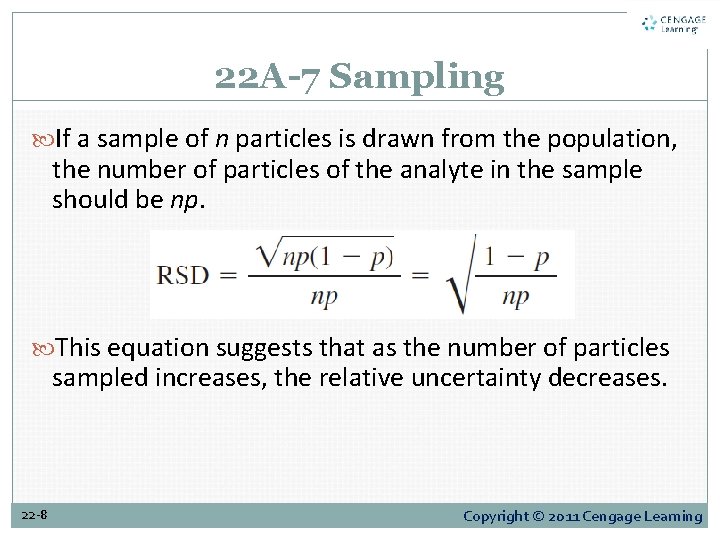
22 A-7 Sampling If a sample of n particles is drawn from the population, the number of particles of the analyte in the sample should be np. This equation suggests that as the number of particles sampled increases, the relative uncertainty decreases. 22 -8 Copyright © 2011 Cengage Learning

For discussion and procedures of each analytical methods, please refer to sections in these chapters. 22 -9 Copyright © 2011 Cengage Learning

THE END 22 -10 Copyright © 2011 Cengage Learning