Introduction to Acids Bases Arrhenius Definition of Acids

Introduction to Acids, Bases
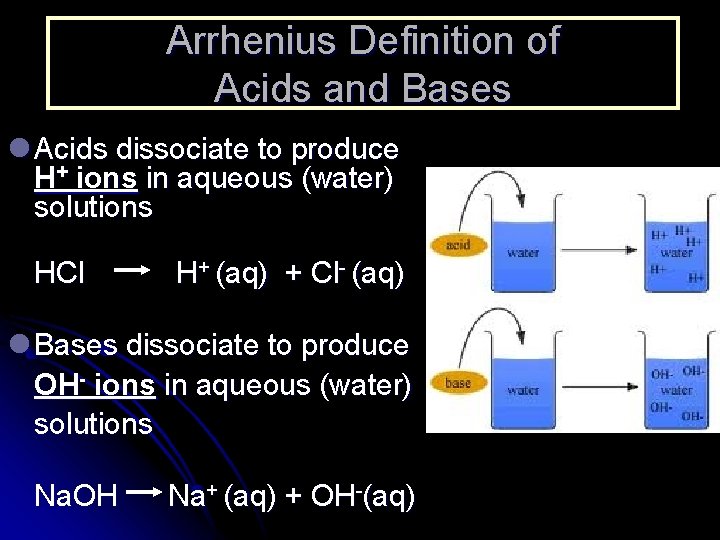
Arrhenius Definition of Acids and Bases l Acids dissociate to produce H+ ions in aqueous (water) solutions HCl H+ (aq) + Cl- (aq) l Bases dissociate to produce OH- ions in aqueous (water) solutions Na. OH Na+ (aq) + OH-(aq)

Dissociation l Dissociation: when a compound splits apart into ions in solution. How might these dissociate? H 2 SO 4 DO NOW: KOH Packet page 3, #7 Practice Writing Dissociation Equations

Note: l Acids and Bases are only reactive if their ions are dissociated (when aqueous) l HCl (l) = hydrogen chloride l HCl (aq) = hydrochloric acid l

Getting to Know Some Acids
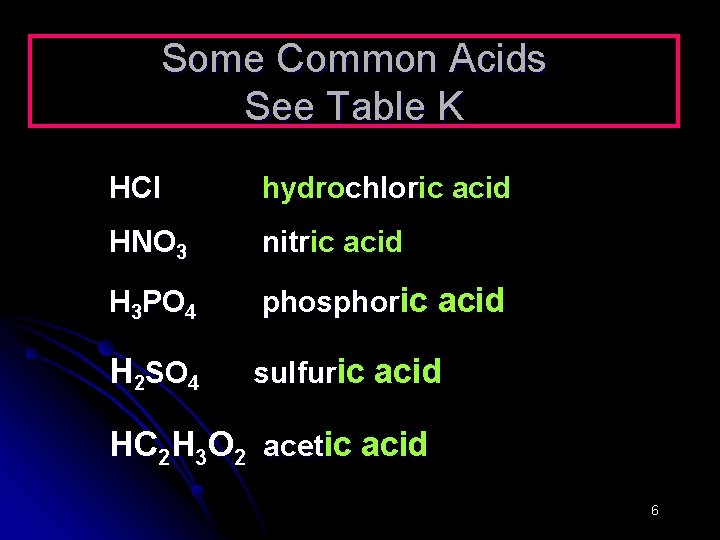
Some Common Acids See Table K HCl hydrochloric acid HNO 3 nitric acid H 3 PO 4 phosphoric acid H 2 SO 4 sulfuric acid HC 2 H 3 O 2 acetic acid 6
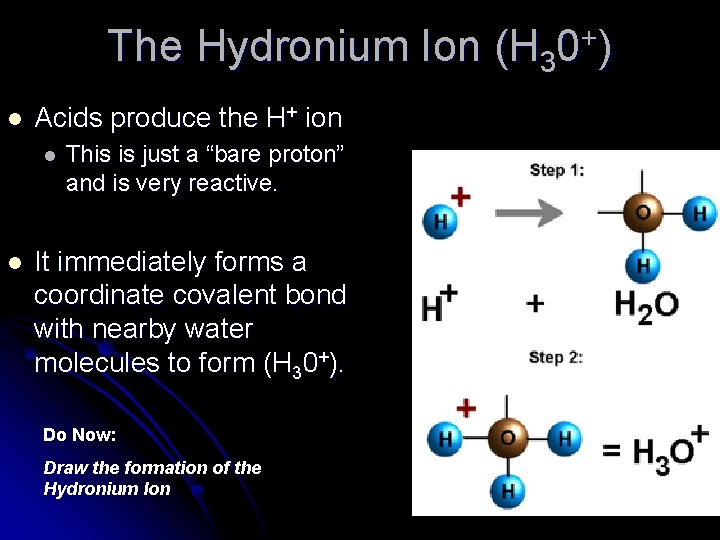
The Hydronium Ion (H 30+) l Acids produce the H+ ion l l This is just a “bare proton” and is very reactive. It immediately forms a coordinate covalent bond with nearby water molecules to form (H 30+). Do Now: Draw the formation of the Hydronium Ion
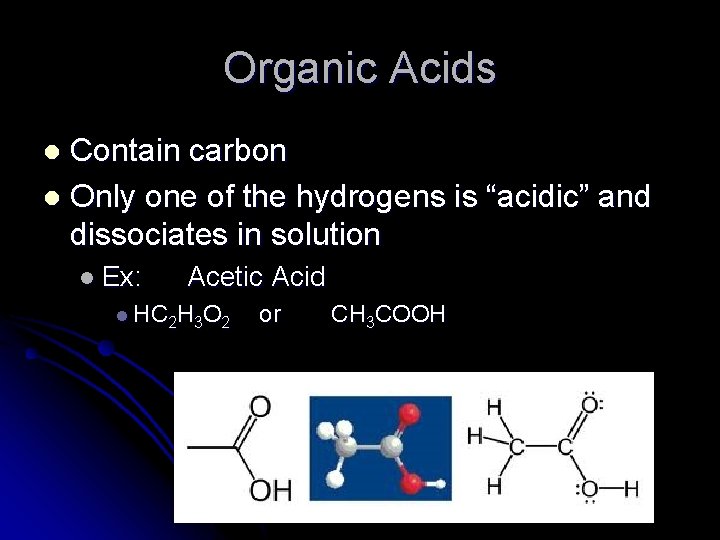
Organic Acids Contain carbon l Only one of the hydrogens is “acidic” and dissociates in solution l l Ex: Acetic Acid l HC 2 H 3 O 2 or CH 3 COOH
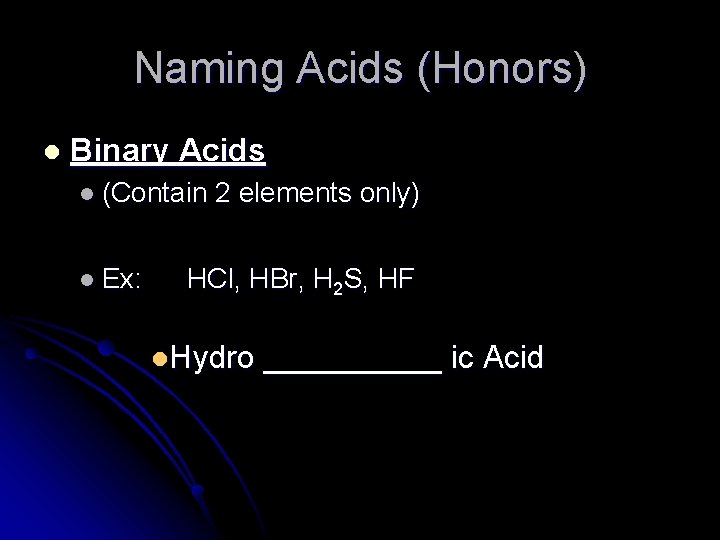
Naming Acids (Honors) l Binary Acids l (Contain 2 elements only) l Ex: HCl, HBr, H 2 S, HF l. Hydro _____ ic Acid

Naming Acids (Honors) l Ternary Acids l (Contain hydrogen and a polyatomic ion) l Do NOT start with “Hydro” l Look at name of polyatomic ion l If it ends in “ate” the acid ends in “ic” l If it ends in “ite” the acid ends in “ous”
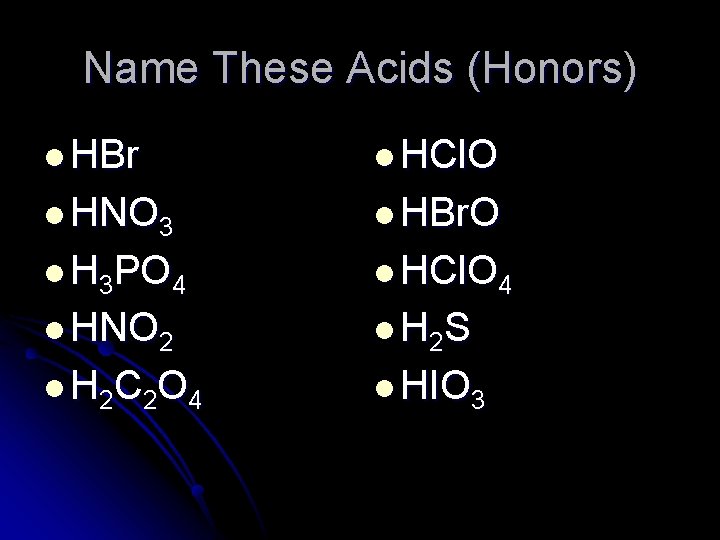
Name These Acids (Honors) l HBr l HCl. O l HNO 3 l HBr. O l H 3 PO 4 l HCl. O 4 l HNO 2 l H 2 S l H 2 C 2 O 4 l HIO 3

Name These Acids (Honors) HBr = hydrobromic acid HCl. O = hypochlorous acid HNO 3 = nitric acid HBr. O = hypobromous acid H 3 PO 4 = phosphoric acid HCl. O 4 = perchloric acid HNO 2 = nitrous acid H 2 S = hydrosulfuric acid H 2 C 2 O 4 = oxalic acid HIO 3 = iodic acid

Getting to Know Some Bases

Naming Bases l All Arrhenius bases contain the hydroxide ion l Name ends in “hydroxide” l Ex: Li. OH = lithium hydroxide l Note: l There later are “Non” Arrhenius Bases, more on them

Some Common Bases See Table L Na. OH sodium hydroxide KOH potassium hydroxide Ba(OH)2 barium hydroxide Mg(OH)2 magnesium hydroxide Al(OH)3 aluminum hydroxide 15
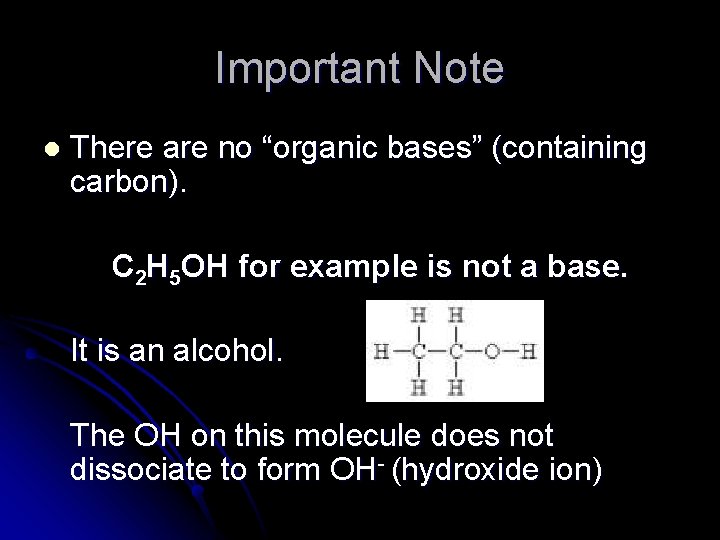
Important Note l There are no “organic bases” (containing carbon). C 2 H 5 OH for example is not a base. It is an alcohol. The OH on this molecule does not dissociate to form OH- (hydroxide ion)

Salts l “Salts” are ionic compounds that are not acids or bases. l Metal cation (+) & nonmetal anion (-) l Ex: Na. Cl, Mg. SO 4, Li 2 S
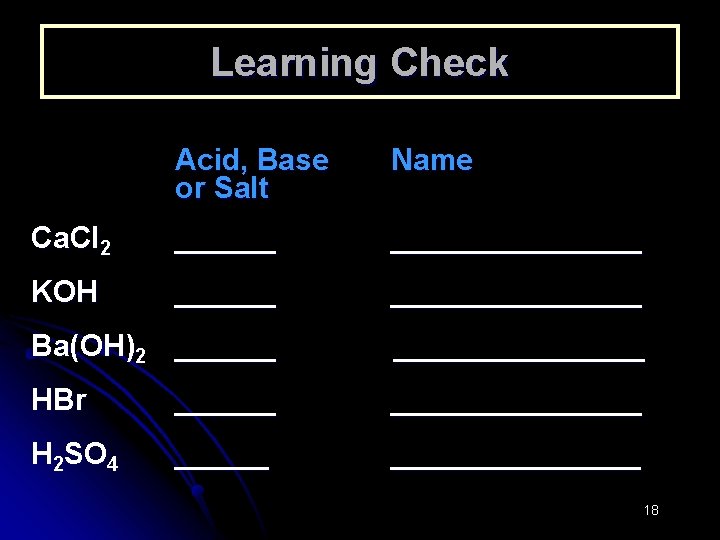
Learning Check Acid, Base or Salt Name Ca. Cl 2 ___________ KOH ___________ Ba(OH)2 ___________ HBr ___________ H 2 SO 4 ___________ 18
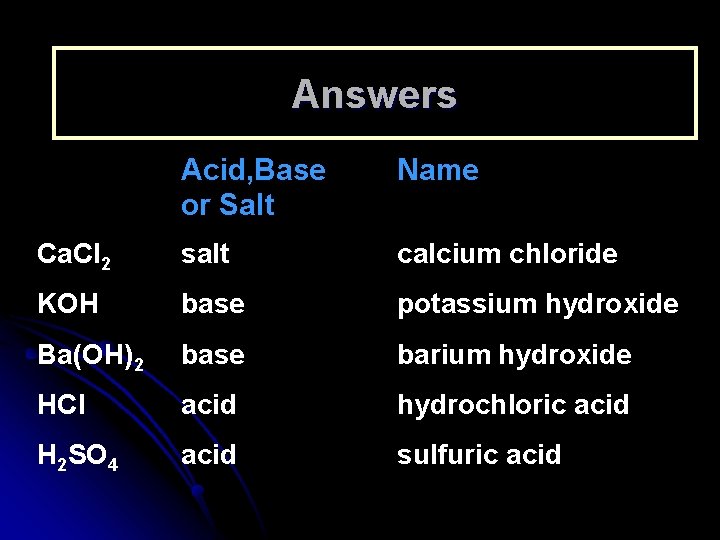
Answers Acid, Base or Salt Name Ca. Cl 2 salt calcium chloride KOH base potassium hydroxide Ba(OH)2 base barium hydroxide HCl acid hydrochloric acid H 2 SO 4 acid sulfuric acid

Electrolytes l Acids & Bases & Salts are electrolytes l Produce free ions when dissolved l Solutions will conduct. l More concentrated = more conductive. Do Now: Practice Ditto on Identifying Electrolytes http: //www. kentchemistry. com/links/A cids. Bases/Electrolytes. htm

Taste & Feel l Acids: taste sour and give a burning sensation if touched l Bases: taste bitter and feel slippery if touched DO NOT attempt to determine an acid or base by taste or touch ever in the lab!

Let’s Review l Which substance, when dissolved in water, forms a solution that conducts an electric current? (1) C 2 H 5 OH (3) C 12 H 22 O 11 (2) C 6 H 12 O 6 (4) CH 3 COOH

A solid substance was tested in the laboratory. The test results are listed below. • dissolves in water • is an electrolyte • melts at a high temperature Based on these results, the solid substance could be (1) Cu (3) C (2) Cu. Br 2 (4) C 6 H 12 O 6

The compound HNO 3 can be described as an (1) Arrhenius acid an electrolyte (2) Arrhenius acid and a nonelectrolyte (3) Arrhenius base and an electrolyte (4) Arrhenius base and a nonelectrolyte

l Based on Reference Table F, which of these salts is the best electrolyte? A. sodium nitrate B. magnesium carbonate C. silver chloride D. barium sulfate

l A substance that conducts an electrical current when dissolved in water is called (1) a catalyst (2) a non-electrolyte (3) a metalloid (4) an electrolyte

l Which compound is an Arrhenius acid? (1) Ca. O (2) K 2 O (3) HCl (4) NH 3

l When one compound dissolves in water, the only positive ion produced in the solution is H 3 O+(aq). This compound is classified as (1) a salt (2) a hydrocarbon (3) an Arrhenius acid (4) an Arrhenius base

l Which substance is an Arrhenius acid? (1) Ba(OH)2 (2) H 3 PO 4 (3) CH 3 COOCH 3 (4) Na. Cl
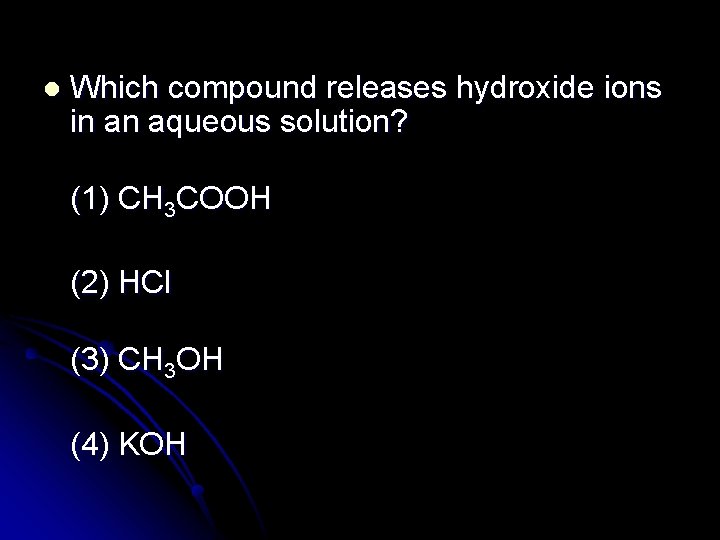
l Which compound releases hydroxide ions in an aqueous solution? (1) CH 3 COOH (2) HCl (3) CH 3 OH (4) KOH

l An Arrhenius base yields which ion as the only negative ion in an aqueous solution? (1) hydride ion (2) hydronium ion (3) hydrogen ion (4) hydroxide ion

l The compound Na. OH(s) dissolves in water to yield (1) hydroxide ions as the only negative ions (2) hydroxide ions as the only positive ions (3) hydronium ions as the only negative ions (4) hydronium ions as the only positive ions

How are HNO 3(aq) and CH 3 COOH(aq) similar? (1) They are Arrhenius acids and they turn blue litmus red. (2) They are Arrhenius acids and they turn red litmus blue. (3) They are Arrhenius bases and they turn blue litmus red. (4) They are Arrhenius bases and they turn red litmus blue.
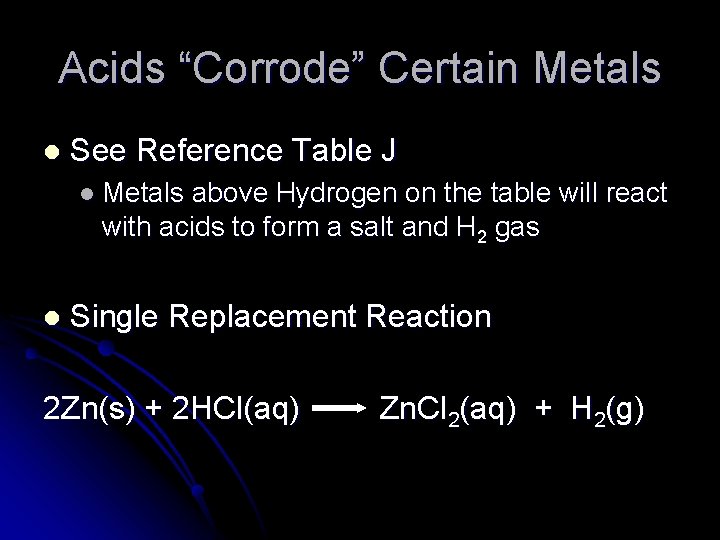
Acids “Corrode” Certain Metals l See Reference Table J l Metals above Hydrogen on the table will react with acids to form a salt and H 2 gas l Single Replacement Reaction 2 Zn(s) + 2 HCl(aq) Zn. Cl 2(aq) + H 2(g)
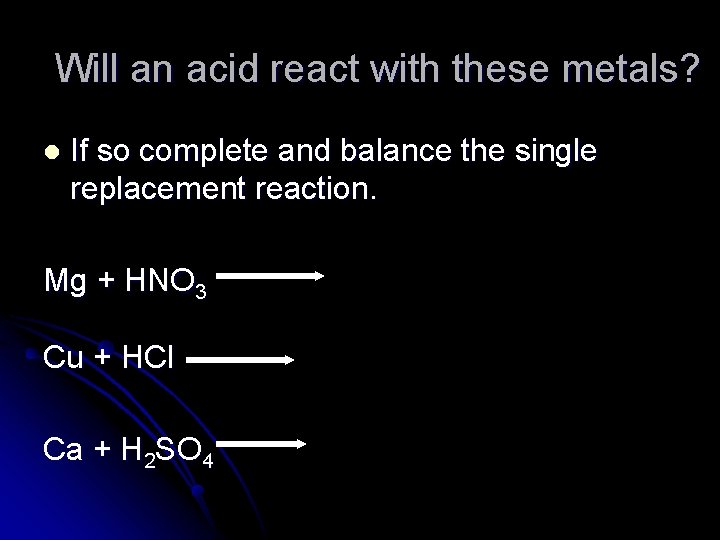
Will an acid react with these metals? l If so complete and balance the single replacement reaction. Mg + HNO 3 Cu + HCl Ca + H 2 SO 4
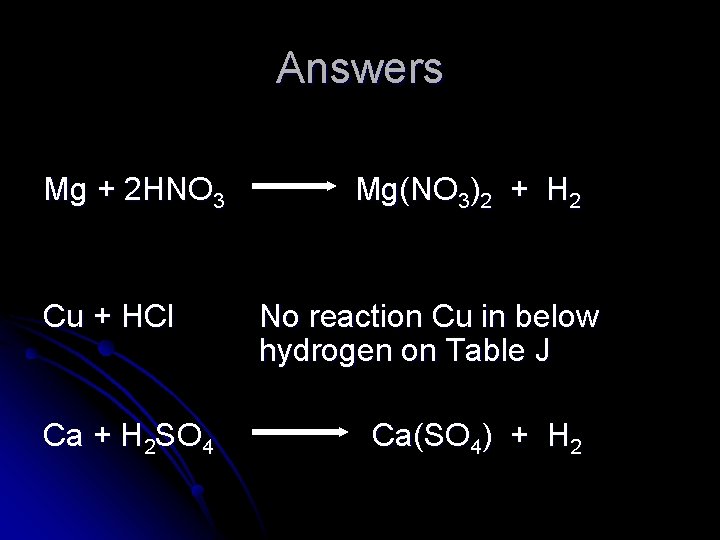
Answers Mg + 2 HNO 3 Cu + HCl Ca + H 2 SO 4 Mg(NO 3)2 + H 2 No reaction Cu in below hydrogen on Table J Ca(SO 4) + H 2

l Bases DO NOT corrode metals DO NOW: Packet page 2 and 3, # 4, #5 l l l Interactive: Acid, Bases and Metals BBC (good for Indicators) http: //www. bbc. co. uk/schools/ks 3 bitesize/science/chemical_material _behaviour/acids_bases_metals/activity. shtml
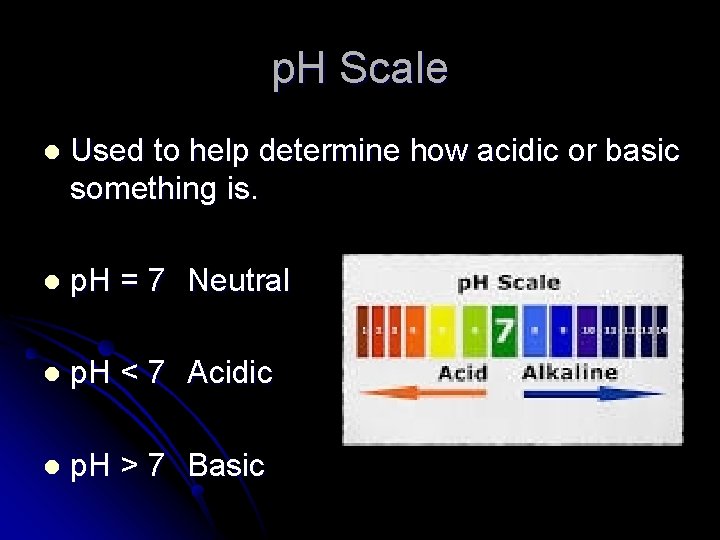
p. H Scale l Used to help determine how acidic or basic something is. l p. H = 7 Neutral l p. H < 7 Acidic l p. H > 7 Basic

Acids & Bases Neutralize Each Other l H+ ion and OH- ions will join together to form neutral water. l Reaction is slightly exothermic (See Table I) H+ (aq) + OH- (aq) → H 2 O (l)

Determining if it is an Acid or Base l How can you tell if something is acidic or basic?

l Use an electronic p. H meter

l Use an indicator such as litmus
l Use p. H paper containing universal indicator
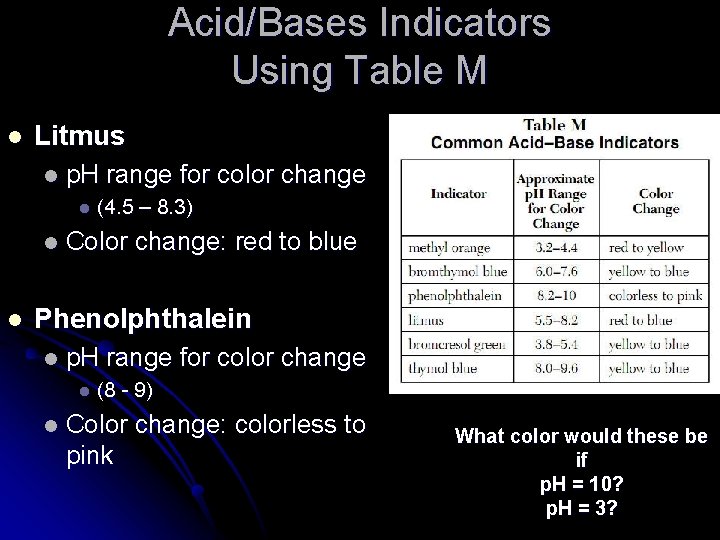
Acid/Bases Indicators Using Table M l Litmus l p. H range for color change l l l (4. 5 – 8. 3) Color change: red to blue Phenolphthalein l p. H range for color change l l (8 - 9) Color change: colorless to pink What color would these be if p. H = 10? p. H = 3?

Let’s Review Based on the results of testing colorless solutions with indicators, which solution is most acidic? (1) a solution in which bromthymol blue is blue (2) a solution in which bromcresol green is blue (3) a solution in which phenolphthalein is pink (4) a solution in which methyl orange is red

l According to Reference Table M, what is the color of the indicator methyl orange in a solution that has a p. H of 2? (1) blue (2) orange (3) yellow (4) red

l Which indicator would best distinguish between a solution with a p. H of 3. 5 and a solution with a p. H of 5. 5? (1) bromthymol blue (2) litmus (3) bromcresol green (4) thymol blue

l Which indicator is blue in a solution that has a p. H of 5. 6? (1) bromcresol green (2) methyl orange (3) bromthymol blue (4) thymol blue

l Which indicator is yellow in a solution with a p. H of 9. 8? (1) methyl orange (2) bromcresol green (3) bromthymol blue (4) thymol blue

l In which solution will thymol blue indicator appear blue? (1) 0. 1 M CH 3 COOH (2) 0. 1 M HCl (3) 0. 1 M KOH (4) 0. 1 M H 2 SO 4
Properties of Acids (Summary) þ Produce H+ (as H 3 O+) ions in water þ Electrolytes (conduct in solution) þ Taste sour þ p. H is < 7 þ Corrode metals (see Table J) þ React with bases to form salts and water (Neutralization)
Properties of Bases (Summary) l. Produce OH- ions in water l Electrolytes (conduct in solution) l Taste bitter, chalky l p. H is >7 l Feel soapy, slippery l React with acids to form salts and water (Neutralization)

Learning Check Describe the solution in each of the following as: 1) acid 2) base or 3)neutral. A. ___soda B. ___soap C. ___coffee D. ___ wine E. ___ water F. ___ grapefruit 53

Solution Describe each solution as: 1) acid 2) base or 3) neutral. A. _1_ soda B. _2_ soap C. _2_ coffee D. _1_ wine E. _3_ water F. _1_ grapefruit 54
Learning Check Identify each as characteristic of an A) acid or B) base ____ 1. Sour taste ____ 2. ____ 3. ____ 4. ____ 5. Produces OH- in aqueous solutions Chalky taste Is an electrolyte Produces H+ in aqueous solutions 55

Solution Identify each as a characteristic of an A) acid or B) base _A_ 1. Sour taste _B_ 2. Produces OH- in aqueous solutions _B_ 3. Chalky taste A, B 4. Is an electrolyte _A_ 5. Produces H+ in aqueous solutions 56
- Slides: 56