INTRODUCTION TO ACIDS BASES AND PH PROPERTIES OF

INTRODUCTION TO ACIDS, BASES AND PH

PROPERTIES OF ACIDS AND BASES Acids are traditionally considered any chemical compound that, when dissolved in water, gives a solution with a hydrogen ion activity greater than in pure water, i. e. a p. H less than 7. 0. They also: Taste sour Are good conductors of electricity (they release H+ ions when they are in water) React with compounds that contain carbonate Are generally quite reactive Turns litmus red
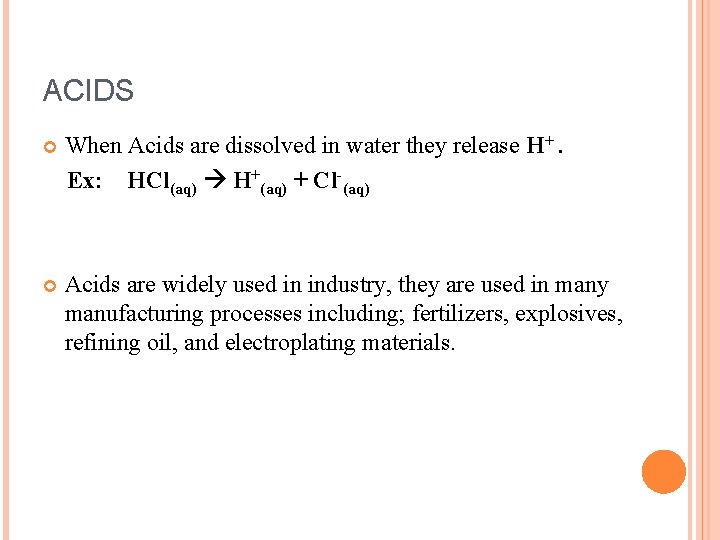
ACIDS When Acids are dissolved in water they release H+. Ex: HCl(aq) H+(aq) + Cl (aq) Acids are widely used in industry, they are used in many manufacturing processes including; fertilizers, explosives, refining oil, and electroplating materials.
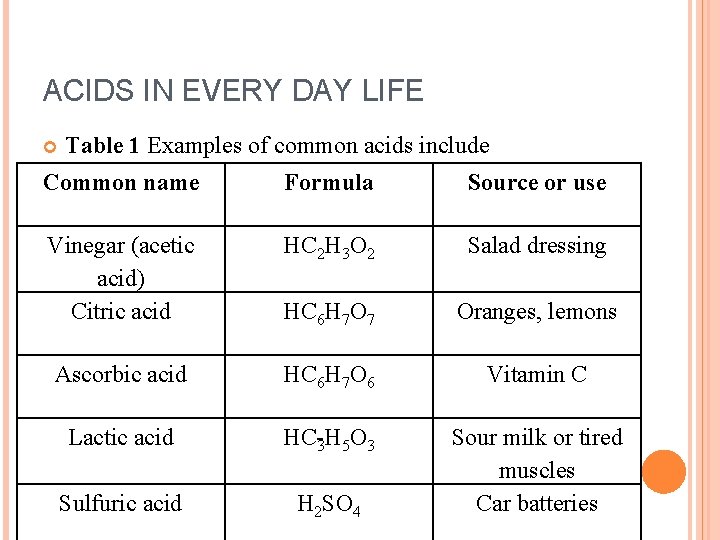
ACIDS IN EVERY DAY LIFE Table 1 Examples of common acids include Common name Formula Source or use Vinegar (acetic acid) Citric acid HC 2 H 3 O 2 Salad dressing HC 6 H 7 O 7 Oranges, lemons Ascorbic acid HC 6 H 7 O 6 Vitamin C Lactic acid HC 3 H 5 O 3 Sulfuric acid H 2 SO 4 Sour milk or tired muscles Car batteries

BASES A base is most commonly thought of as an aqueous substance that can accept hydrogen ions. bases can commonly be thought of as any chemical compound that, when dissolved in water, gives a solution with a hydrogen ion activity lower than that of pure water, i. e. a p. H higher than 7. 0 at standard conditions. They also:

PROPERTIES OF BASES: Taste bitter Are good conductors of electricity (They release OH ions when dissolved in water) Break down proteins into smaller molecules May also be called alkaline Turns litmus blue

BASES When bases are released in water they release OH ions Example Na. OH (aq) Na+ (aq) + OH (aq)
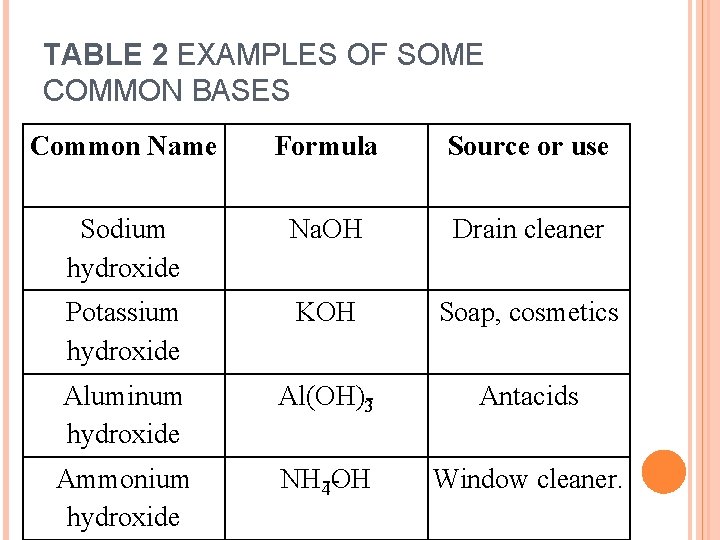
TABLE 2 EXAMPLES OF SOME COMMON BASES Common Name Formula Source or use Sodium hydroxide Na. OH Drain cleaner Potassium hydroxide KOH Soap, cosmetics Aluminum hydroxide Al(OH) 3 Antacids Ammonium hydroxide NH 4 OH Window cleaner.

FORMULAS FOR ACIDS AND BASES Acids Common acids can be recognised because their formula begins with (H) hydrogen. Examples: H 2 SO 4 H 2 CO 3 H 3 PO 4 HCl
BASES Bases are not as easy to recognise, most will contain a hydroxyl group (OH ). Ex: Na. OH, Mg(OH)2 Other compounds can form bases when they react with water to form OH ions. Compounds that contain carbonate (CO 32 ) or bicarbonate (HCO 3 ) react
THE PH SCALE The p. H scale is used to represent how acid or basic a solution is. The scale ranges from 0 14 with very acidic being 0, neutral being 7, and very basic being 14. Every point on the scale represents a 10 base exponent difference. Ex lemons (p. H = 2. 0) are 100 times more acidic than tomatoes (p. H = 4. 0) 7. 0 is neutral (neither acidic nor basic (alkaline)). Acids range from 0 6. 9 Bases range from 7. 1 14
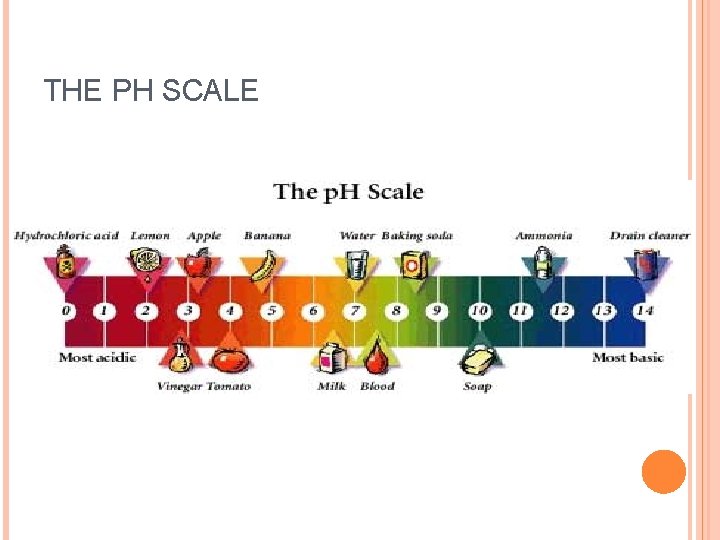
THE PH SCALE
- Slides: 12