Introduction Titanium and its Alloys Titanium is named




















- Slides: 63

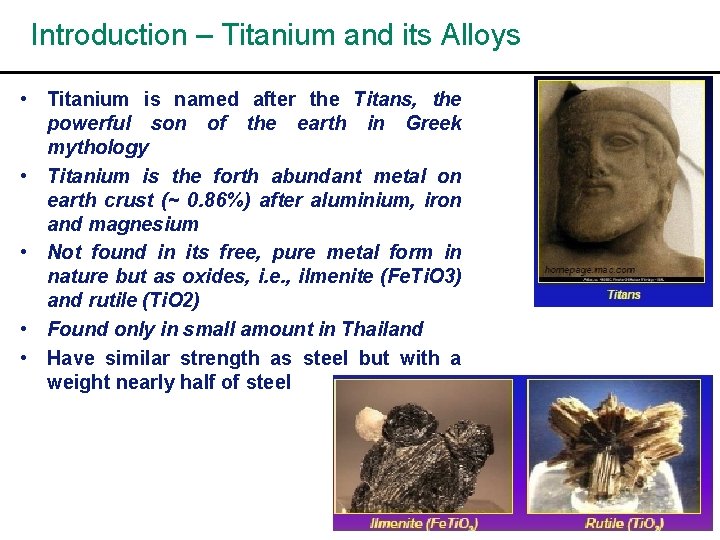
Introduction – Titanium and its Alloys • Titanium is named after the Titans, the powerful son of the earth in Greek mythology • Titanium is the forth abundant metal on earth crust (~ 0. 86%) after aluminium, iron and magnesium • Not found in its free, pure metal form in nature but as oxides, i. e. , ilmenite (Fe. Ti. O 3) and rutile (Ti. O 2) • Found only in small amount in Thailand • Have similar strength as steel but with a weight nearly half of steel

History of Titanium ( In 1789 Rev. W. Gregor discovers Titanium dioxide in Rutile. a 1795 Klaporth rediscovers titanium dioxide and recognizes it as the oxide of a metal to which he gives the name titanium. , 1821 -3 Rose reports on titanium, its oxides and sulphides. These were the first reports on pure compounds of this element. , 1825 Berzelius isolates metallic titanium. , 1869 Schonn reports yellow color produced by reacting titanium salts with hydrogen peroxide.

Physical Properties of Titanium • Experiences allotropic transformation (α → β) at 882. 5 C. • Highly react with oxygen, nitrogen, carbon and hydrogen. • Difficult to extract → expensive. • Used mainly in wrought forms for advanced applications where cost is not critical. • High strength and toughness.

Description of Titanium Z K K K Titanium, when pure, is a lustrous, white metal. Titanium minerals are quite common. The metal has a low density (4. 5 gm/cm 3) compared to Al (2. 69 gm/cm 3, Cu 8. 96 gm/cm 3, Fe 7. 88 gm/cm 3) Superior strength to weight ratio. It is twice as strong as aluminum. High temperature strength (up to 650 C). Can be easily fabricated has excellent corrosion resistance. It is nearly as resistant to K corrosion as platinum. The metal burns in air and is the only element that burns in nitrogen. It is marvelous in fireworks. K Low electrical and thermal conductivity K

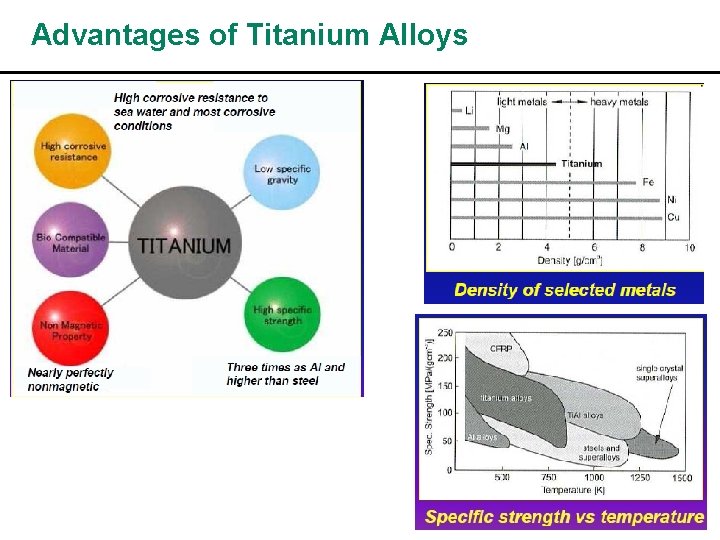
Advantages of Titanium Alloys

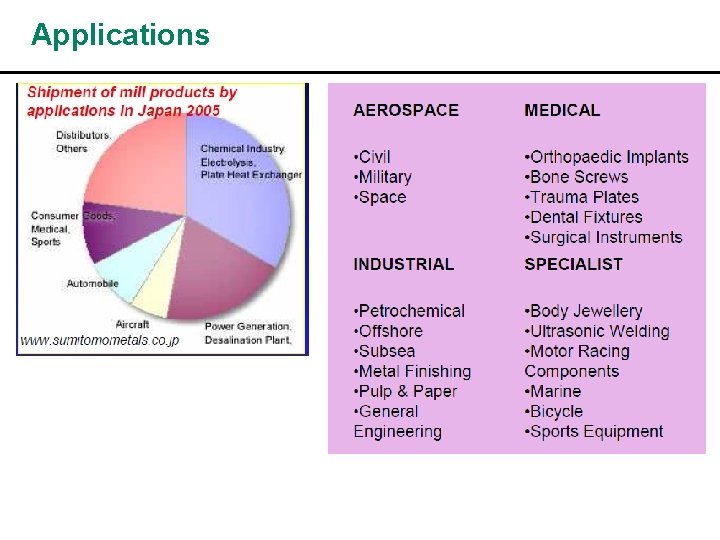
Uses of Titanium is used for alloys with aluminum, molybdenum, manganese, iron, and other metals. > These alloys of titanium are used principally in the aerospace industry, for both airframes and engines, where lightweight strength and ability to withstand extremes of temperature are important. > Its excellent corrosion resistance provides applications in chemical processing equipments, marine components and biomedical implants like joint replacement parts, including hip ball and sockets. >

Uses of Titanium cont. J n J J It has excellent resistance to sea water and is used for propeller shafts, rigging, and other parts of ships exposed to salt water. Titanium alloys are considered biocompatible (i. e. , they are not rejected by the body). By developing porous coatings of bone-like ceramic compositions known as hydroxyapatite, it may be possible to make titanium implants bioactive (i. e. , the natural bone can grow into the hydroxyapatite coating). A titanium anode coated with platinum provides cathodic protection from corrosion by salt water. Titanium paint is an excellent reflector of infrared radiation, and is extensively used in solar observatories where heat causes poor viewing conditions.

Continued A A Pure titanium dioxide is relatively clear and has an extremely high index of refraction with an optical dispersion higher than diamond. It is produced artificially for use as a gemstone, but it is relatively soft. Star sapphires and rubies exhibit their asterism as a result of the presence of Ti. O 2. The dioxide is used extensively for paint as it is permanent and has good covering power.

Applications

Applications

Applications

Applications

Info on Titanium is resistant to dilute sulphuric and hydrochloric acid, most organic acids, chlorine gas, and chloride solutions. Titanium is present in meteorites and in the sun. Some lunar rocks contain high concentrations of the dioxide, Ti. O 2. þ Titanium is widely distributed in the universe. Its concentration within the earth’s crust is of about 0. 6% makes it the fourth most abundant of metals after Al, Fe and Mg þ Pure Ti melts at 1670 C – ideal to use component for elevated temperature þ

Reactions è The picture shows the result from adding titanium powder to a burning mixture of potassium chlorate and sucrose. Do not attempt this reaction unless are a professionally qualified chemist and you have carried out a legally satisfactory hazard assessment.

More Reactions g This picture shows the reaction between titanium metal and potassium perchlorate (KCl. O 4). Improperly done, this reaction is dangerous!

Fun Facts titane means Titanium in French. &Titan means Titanium in German. ) titanio means Titanium in Italy. < Titânio means Titanium in Portuguese. ó titanio means Titanium in Spanish. Ø Titan means Titanium in Sweden. i

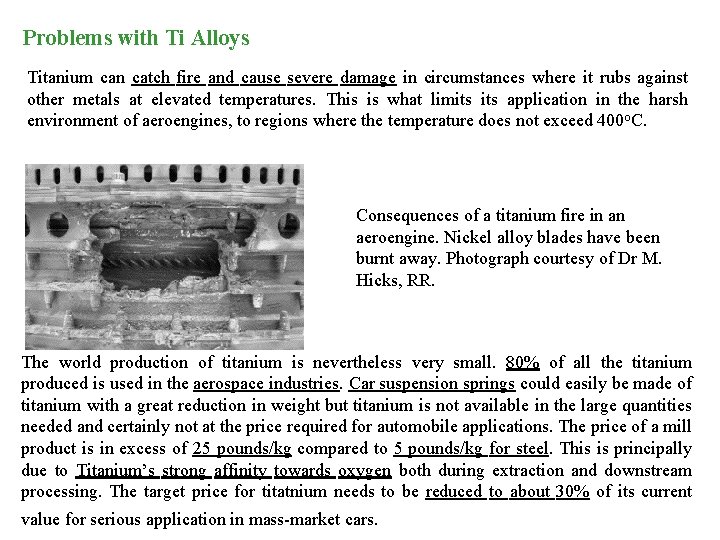
Problems with Ti Alloys Titanium can catch fire and cause severe damage in circumstances where it rubs against other metals at elevated temperatures. This is what limits application in the harsh environment of aeroengines, to regions where the temperature does not exceed 400 o. C. Consequences of a titanium fire in an aeroengine. Nickel alloy blades have been burnt away. Photograph courtesy of Dr M. Hicks, RR. The world production of titanium is nevertheless very small. 80% of all the titanium produced is used in the aerospace industries. Car suspension springs could easily be made of titanium with a great reduction in weight but titanium is not available in the large quantities needed and certainly not at the price required for automobile applications. The price of a mill product is in excess of 25 pounds/kg compared to 5 pounds/kg for steel. This is principally due to Titanium’s strong affinity towards oxygen both during extraction and downstream processing. The target price for titatnium needs to be reduced to about 30% of its current value for serious application in mass-market cars.

Production • Reduction of ore to sponge • Melting of sponge to form an ingot • Primary fabrication into billet or bars • Secondary fabrication into finished shapers

Production • Pure metallic Ti (99. 9%) pure was first prepared in 1910 by Matthew A. Hunter by heating Ti. Cl 4 in a steel container at 700 -800 C – known as Hunter process • Ti was not used outside the laboratory till 1946 when Willium Justin Kroll produced it by reducing Ti. Cl 4 with Mg - known as Kroll process

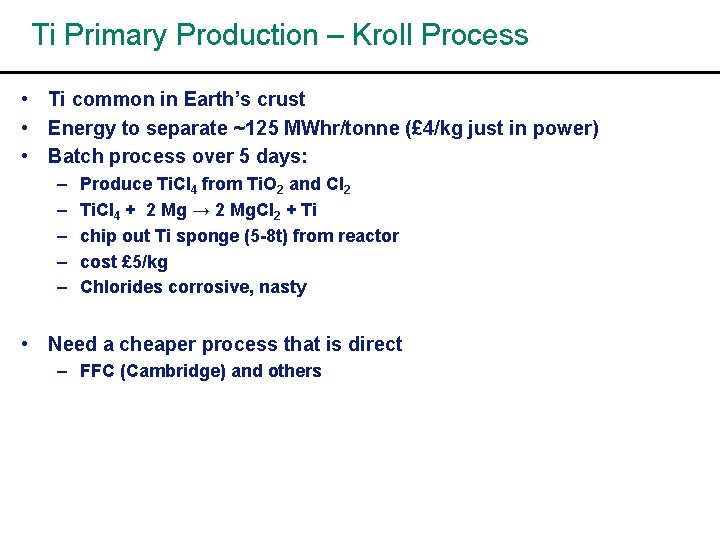
Ti Primary Production – Kroll Process • Ti common in Earth’s crust • Energy to separate ~125 MWhr/tonne (£ 4/kg just in power) • Batch process over 5 days: – – – Produce Ti. Cl 4 from Ti. O 2 and Cl 2 Ti. Cl 4 + 2 Mg → 2 Mg. Cl 2 + Ti chip out Ti sponge (5 -8 t) from reactor cost £ 5/kg Chlorides corrosive, nasty • Need a cheaper process that is direct – FFC (Cambridge) and others

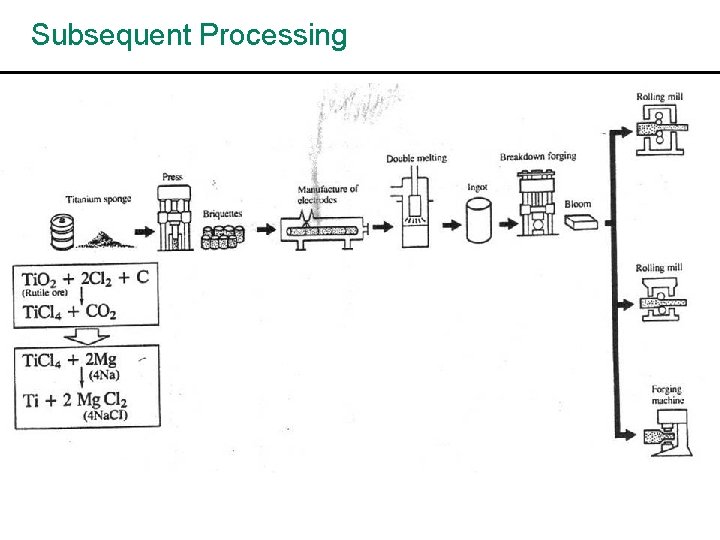
Subsequent Processing harvey fig p 11

Crystal Structure of Ti n Alpha α : (room temperature to 8830 C) - Hexagonal close packed (HCP) - Usually strong but brittle - Not easy to form into various shape n Beta β: (>8830 C) - Body Centered Cubic (BCC, the same as steel at RT) - Less strong but not so brittle - Slightly easier to form into different shapes The properties of commercially pure titanium (99 -99. 5%) are largely determined by the oxygen content.

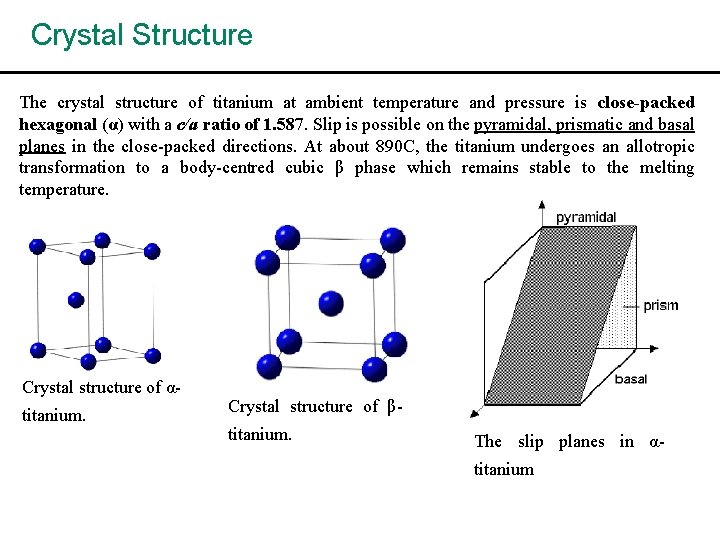
Crystal Structure The crystal structure of titanium at ambient temperature and pressure is close-packed hexagonal (α) with a c/a ratio of 1. 587. Slip is possible on the pyramidal, prismatic and basal planes in the close-packed directions. At about 890 C, the titanium undergoes an allotropic transformation to a body-centred cubic β phase which remains stable to the melting temperature. Crystal structure of αtitanium. Crystal structure of βtitanium. The slip planes in αtitanium

Alloying of Titanium All elements which are within the range 0. 85 -1. 15 of the atomic radius of titanium alloy dissolve substitutionally and have a significant solubility in titanium. • • Elements with an atomic radius less than 0. 59 that of Ti occupy interstitial sites and also have Substantial solubility (e. g. H, N, O, C). • The ease with which solutes dissolve in titanium makes it difficult to design precipitation-hardened alloys. • Boron has a similar but larger radius than C, O, N and H; it is therefore possible to induce titanium boride precipitation. Copper precipitation is also possible in appropriate alloys.

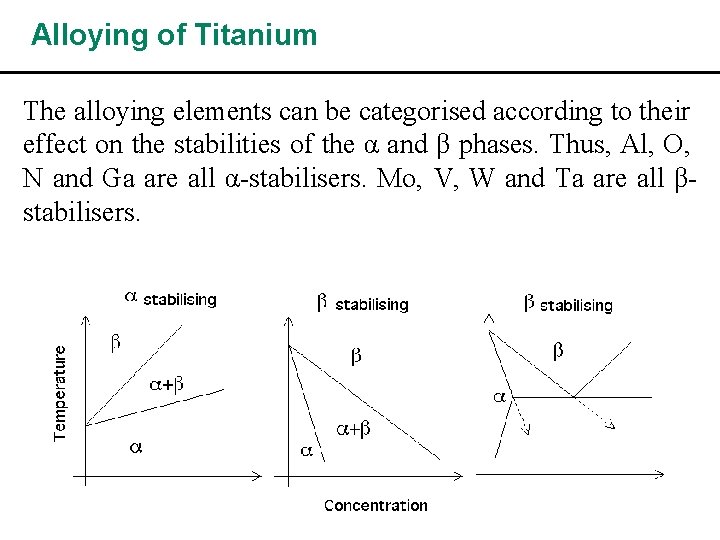
Alloying of Titanium The alloying elements can be categorised according to their effect on the stabilities of the α and β phases. Thus, Al, O, N and Ga are all α-stabilisers. Mo, V, W and Ta are all βstabilisers.

Alloying of Titanium • Molybdenum and vanadium have the largest influence on β stability and are common alloying elements. Tungsten is rarely added due to its high density. Cu forms Ti. Cu 2 which makes the alloys age-hardening and heat treatable; such alloys are used as sheet materials. It is typically added in concentrations less than 2. 5 wt% in commercial alloys. • Zr, Sn and Si are neutral elements. These do not fit properly and cause changes in the lattice parameters. • Hydrogen is the most important interstitial. Body-centred cubic Ti has three octahedral interstices per atom whereas c. p. h. Ti has one per atom. The latter are therefore larger, so that the solubility of O, N, and C is much higher in the α phase.

Interstitials • Titanium is capable of absorbing up to 60 at. % of hydrogen, which can also be removed by annealing in a vacuum. Hydrogen enters the tetrahedral holes which are larger in b. c. c. than c. p. h. Thus the solubility of hydrogen is larger in β. The enthalpy of solution of hydrogen in Ti is negative (ΔH<0). • Because of this characteristic, titanium is a candidate material for the first wall of magnetically confined fusion reactors. The hydrogen based plasma is not detrimental since at 500 o. C and 1 Pa pressure, the Ti does not pick up enough hydrogen for embrittlement. An additional feature is that Ti resists swelling due to neutron damage.

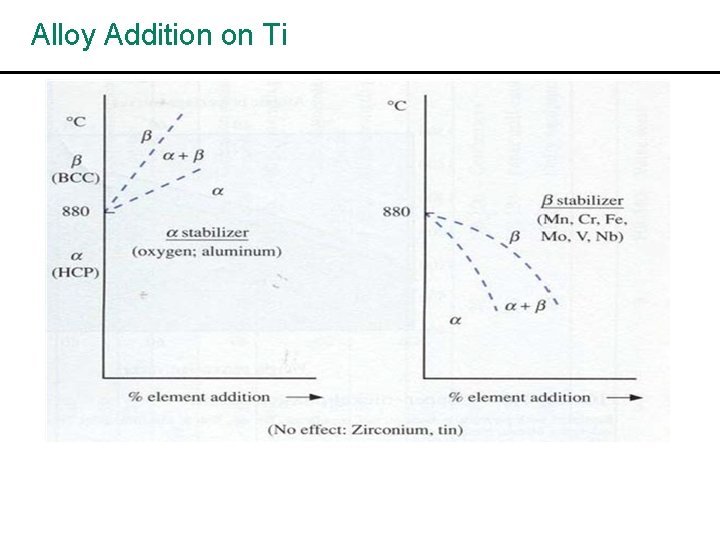
Alloy Addition on Ti

• Addition of AE influences the α to β transformation temperature: α stab – Al, Cu, C, O, N – they raise β stab – Cr, Mo, Mn, V, Fe, Ta • Sn and Zr – Alloyed with Ti and have extensive solid solubility both in α and β. They do not strongly promote phase stability but retard the rate of transformation and useful as strengthening agent. • Ti alloy contain 92. 5% Ti, 5% Al, 2. 5% Sn are strong and maintain their strength at high temp. but are difficult to work. The alloys have good weldability and are used where high temp strength is required, e. g. steam turbine blades. The 90% Ti, 6% Va alloy can be readily welded, forged and machined. This alloy is used for both high and low temp. applications, e. g. rocket motor cases, turbine blades.

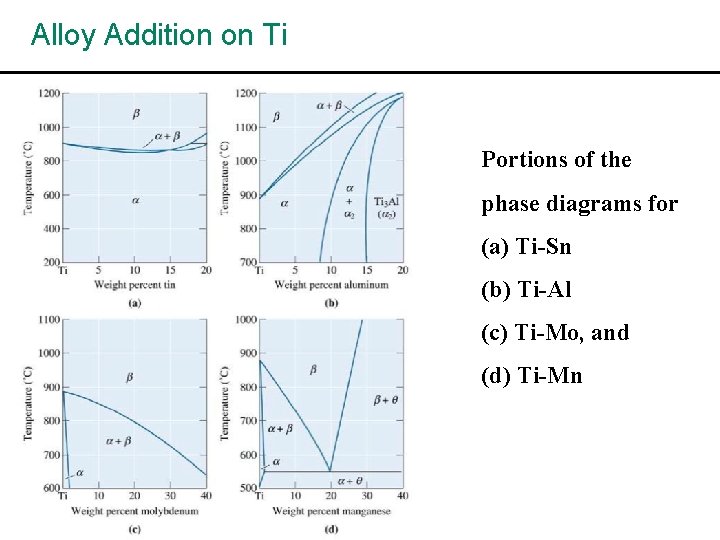
Alloy Addition on Ti Portions of the phase diagrams for (a) Ti-Sn (b) Ti-Al (c) Ti-Mo, and (d) Ti-Mn

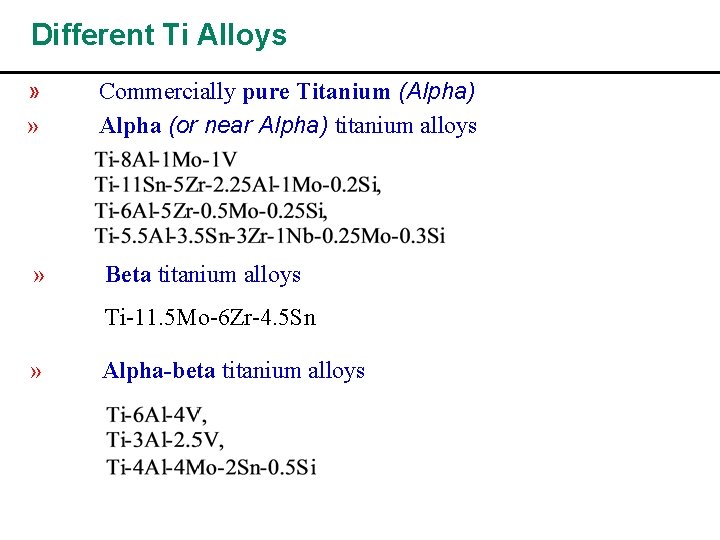
Different Ti Alloys » » Commercially pure Titanium (Alpha) Alpha (or near Alpha) titanium alloys » Beta titanium alloys Ti-11. 5 Mo-6 Zr-4. 5 Sn » Alpha-beta titanium alloys

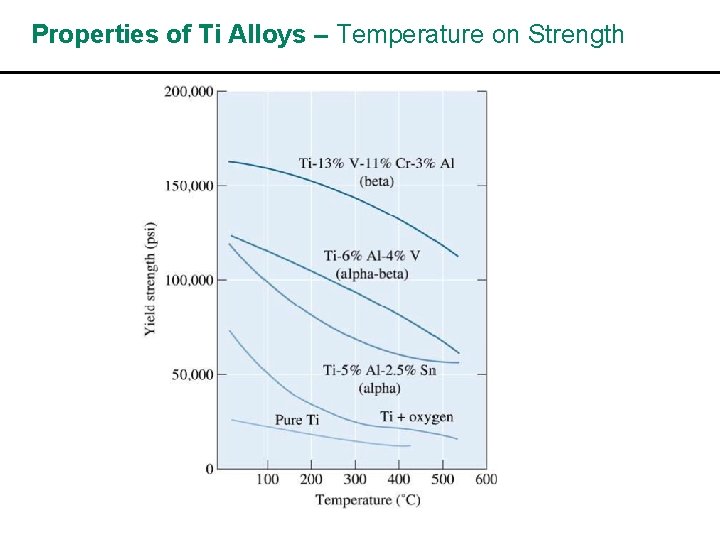
Properties of Ti Alloys – Temperature on Strength

Properties of Ti Alloys – Alpha Ti Alloys • Strong • High strength at high temperatures (<883 o. C) • Good weldability • Difficult to work • Non-heat treatable (stress relieving and annealing) • Tensile strength: 330 -860 MPa • Fracture toughness: >70 MPa m-1/2 • Strengthen pure α alloys by –solid solution – O, Al, Sn –Hall-Petch – σ = 231 + 10. 5√ d –cold work –martensite reaction exists, of little benefit (not heat-treatable)

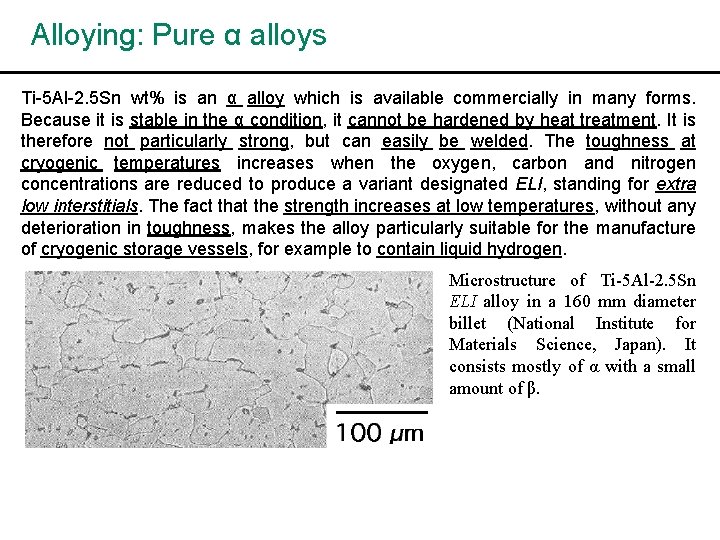
Alloying: Pure α alloys Ti-5 Al-2. 5 Sn wt% is an α alloy which is available commercially in many forms. Because it is stable in the α condition, it cannot be hardened by heat treatment. It is therefore not particularly strong, but can easily be welded. The toughness at cryogenic temperatures increases when the oxygen, carbon and nitrogen concentrations are reduced to produce a variant designated ELI, standing for extra low interstitials. The fact that the strength increases at low temperatures, without any deterioration in toughness, makes the alloy particularly suitable for the manufacture of cryogenic storage vessels, for example to contain liquid hydrogen. Microstructure of Ti-5 Al-2. 5 Sn ELI alloy in a 160 mm diameter billet (National Institute for Materials Science, Japan). It consists mostly of α with a small amount of β.

Properties of Ti Alloys – Near Alpha Ti Alloys • Almost all alpha phase • Small amount of beta phase disperse throughout the alpha • Non-heat treatable (stress relieving and annealing) • Improved creep resistance at temperatures at 450 -500 o. C • Tensile strength: 855 -1040 MPa • Fracture toughness: 50 -70 MPa m-1/2

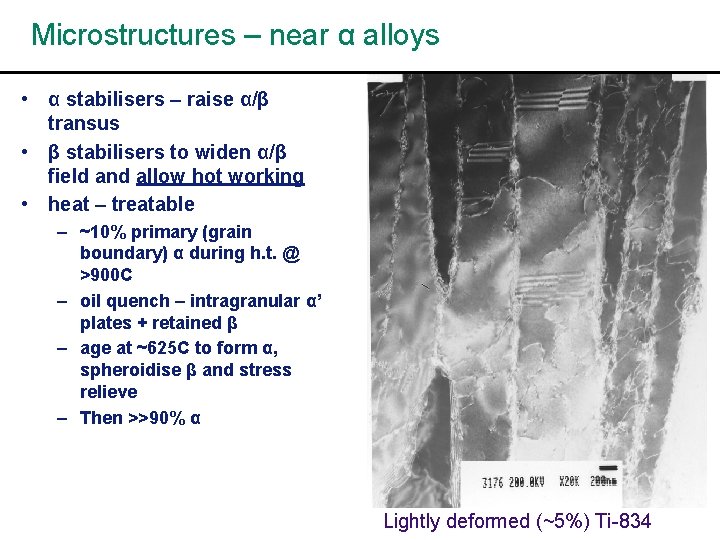
Microstructures – near α alloys • α stabilisers – raise α/β transus • β stabilisers to widen α/β field and allow hot working • heat – treatable – ~10% primary (grain boundary) α during h. t. @ >900 C – oil quench – intragranular α’ plates + retained β – age at ~625 C to form α, spheroidise β and stress relieve – Then >>90% α Lightly deformed (~5%) Ti-834

Microstructures – near α alloys The niobium is added for oxidation resistance and the carbon to allow a greater temperature range over which the alloy is a mixture of α+β, in order to facilitate thermomechanical processing. This particular alloy is used in the manufacture of aeroengine discs and has replaced discs made from much heavier nickel base superalloys. The final microstructure of the alloy consists of equiaxed primary-α grains, Widmanstätten α plates separated by the β-phase.

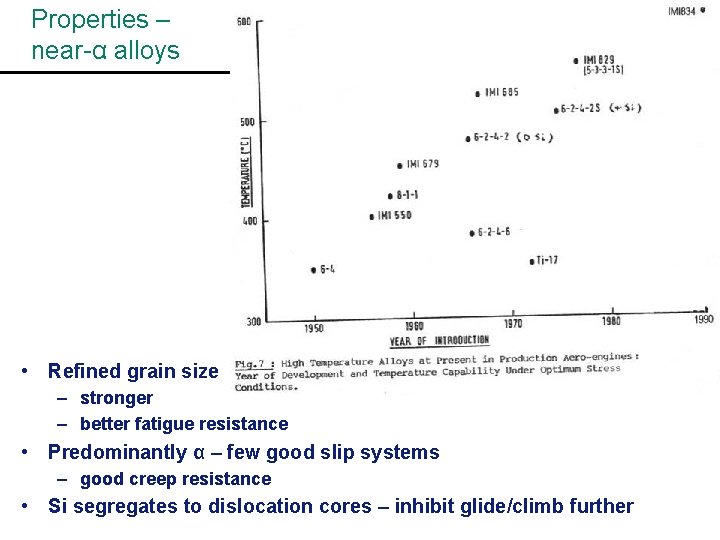
Properties – near-α alloys • Refined grain size – stronger – better fatigue resistance • Predominantly α – few good slip systems – good creep resistance • Si segregates to dislocation cores – inhibit glide/climb further

Properties of Ti Alloys – Beta Ti Alloys • Entirely beta phase at room temperature after quenching (fast cooling), or sometimes even upon air cooling • Ready for cold working (forming) • Can be solution treated, quenched and aged to give higher strength (ageing at elevated temperature after quenching results in decomposition of beta phase leading to strengthening) • In high strength condition the alloys have low ductility • Poor fatigue performance • Tensile strength: 1220 -1450 MPa • Fracture toughness: >50 MPa m-1/2

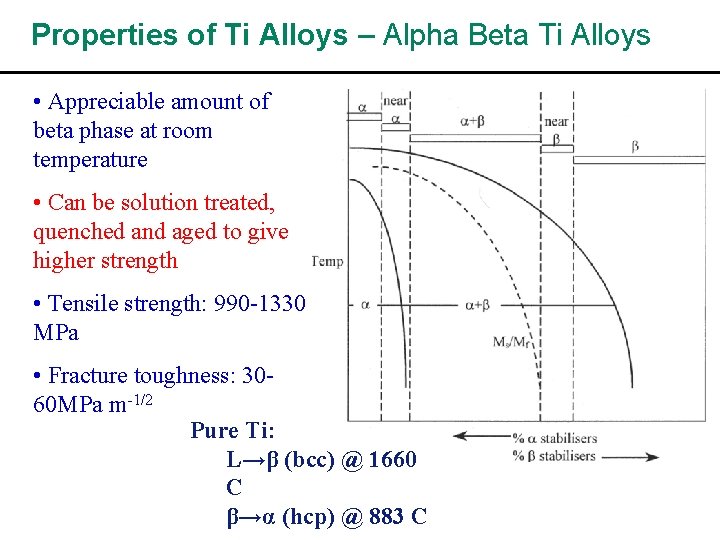
Properties of Ti Alloys – Alpha Beta Ti Alloys • Appreciable amount of beta phase at room temperature • Can be solution treated, quenched and aged to give higher strength • Tensile strength: 990 -1330 MPa • Fracture toughness: 3060 MPa m-1/2 Pure Ti: L→β (bcc) @ 1660 C β→α (hcp) @ 883 C

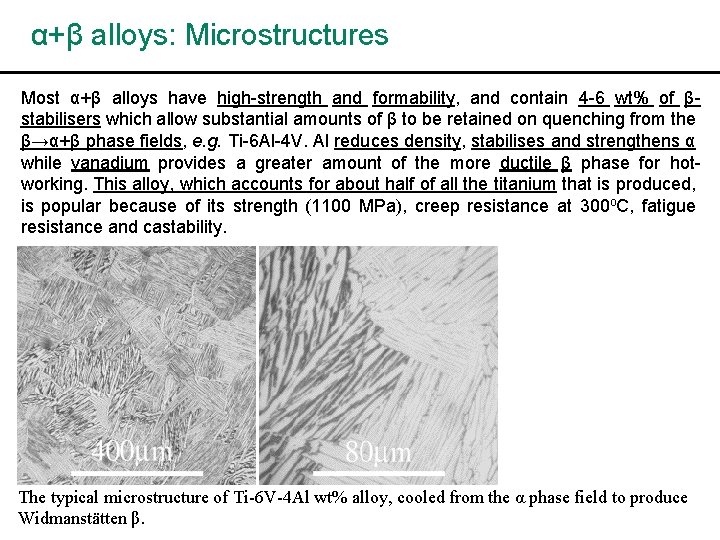
α+β alloys: Microstructures Most α+β alloys have high-strength and formability, and contain 4 -6 wt% of βstabilisers which allow substantial amounts of β to be retained on quenching from the β→α+β phase fields, e. g. Ti-6 Al-4 V. Al reduces density, stabilises and strengthens α while vanadium provides a greater amount of the more ductile β phase for hotworking. This alloy, which accounts for about half of all the titanium that is produced, is popular because of its strength (1100 MPa), creep resistance at 300 o. C, fatigue resistance and castability. The typical microstructure of Ti-6 V-4 Al wt% alloy, cooled from the α phase field to produce Widmanstätten β.

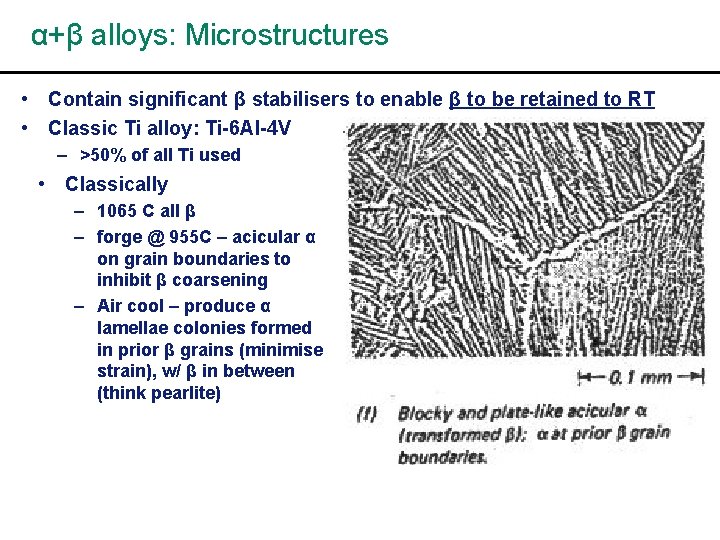
α+β alloys: Microstructures • Contain significant β stabilisers to enable β to be retained to RT • Classic Ti alloy: Ti-6 Al-4 V – >50% of all Ti used • Classically – 1065 C all β – forge @ 955 C – acicular α on grain boundaries to inhibit β coarsening – Air cool – produce α lamellae colonies formed in prior β grains (minimise strain), w/ β in between (think pearlite)

α+β alloys: Microstructures One difficulty with the β phase, which has a body-centred cubic crystal structure, is that like ferritic iron, it has a ductile-brittle transition temperature. The transition temperature tends to be above room temperature, with cleavage fracture dominating at ambient temperatures.

Heat Treatment of Ti Alloys Stress Relieving Annealing Solution treating and ageing (only for β and α-β alloys)

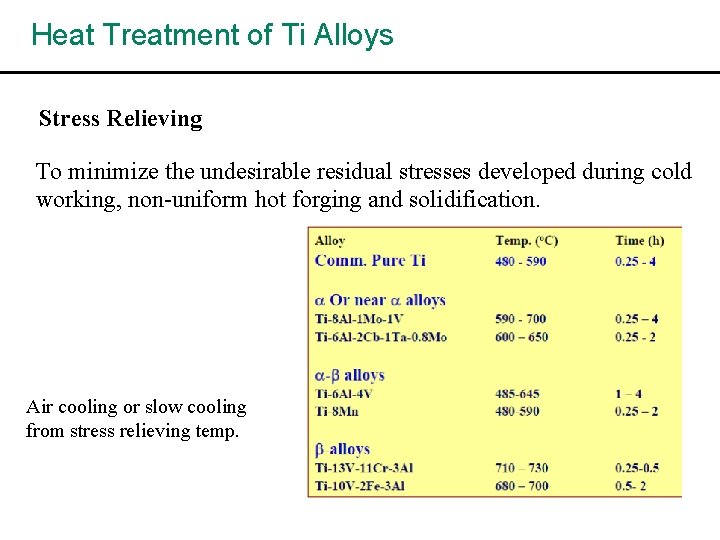
Heat Treatment of Ti Alloys Stress Relieving To minimize the undesirable residual stresses developed during cold working, non-uniform hot forging and solidification. Air cooling or slow cooling from stress relieving temp.

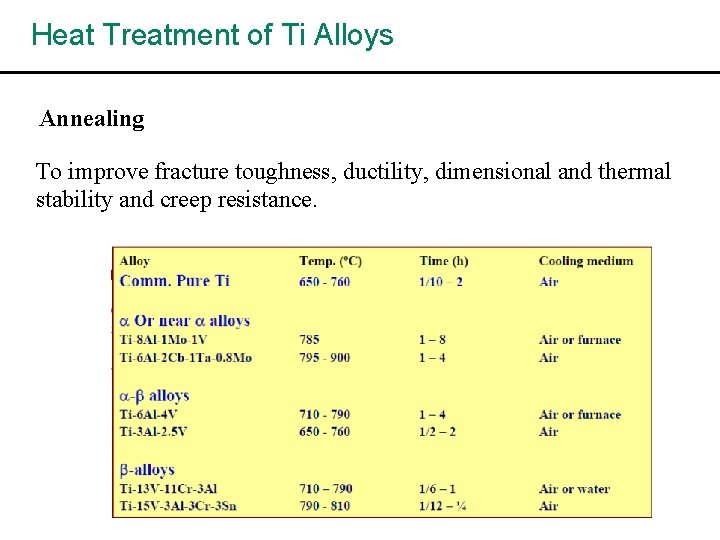
Heat Treatment of Ti Alloys Annealing To improve fracture toughness, ductility, dimensional and thermal stability and creep resistance.

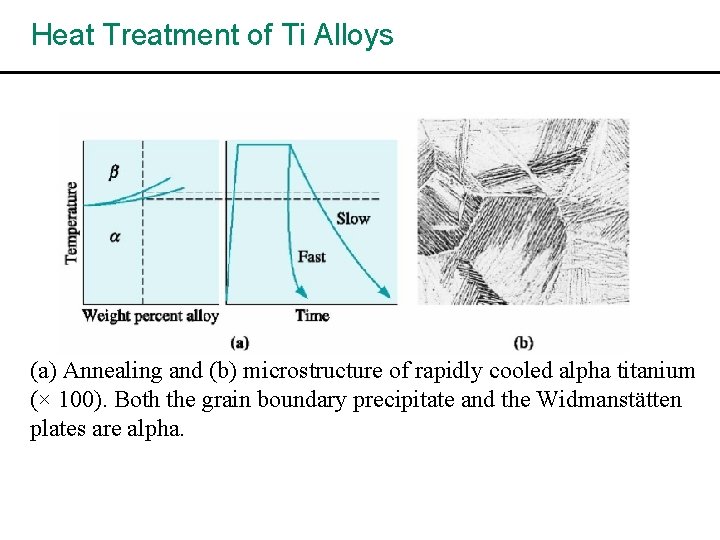
Heat Treatment of Ti Alloys (a) Annealing and (b) microstructure of rapidly cooled alpha titanium (× 100). Both the grain boundary precipitate and the Widmanstätten plates are alpha.

Heat Treatment of Ti Alloys Solution Treating and Ageing High temperature β-phase is unstable at lower temperature. Higher ratio of β is produced by heating an α-β alloy to the solution treating temperature. By quenching, the higher proportion of β phase is maintained. Decomposition of metastable β-phase takes place during subsequent ageing. Generally, β-alloys are in air, whereas α-β alloys are quenched in water (or brine solution) from solution treating temperature. After quenching, artificial ageing treatment is carried out at 430 – 650 C.

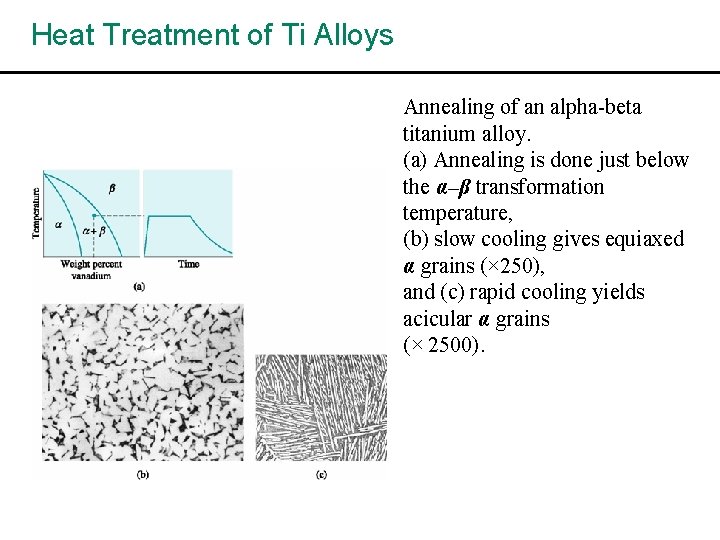
Heat Treatment of Ti Alloys Annealing of an alpha-beta titanium alloy. (a) Annealing is done just below the α–β transformation temperature, (b) slow cooling gives equiaxed α grains (× 250), and (c) rapid cooling yields acicular α grains (× 2500).

Heat Treatment of Ti Alloys Metastable β Transformed to ω (particularly, for highly β stabilized α-β alloys) ω is brittle phase thus should be avoided. This is done by rapid quenching and fast reheating to ageing temperature ( >430 C). Addition of Al prevented ω formation by increasing sluggishness of β to ω transformation. Mo suppresses formation by stabilizing βphase. Ti-6 Al-4 V (α-β alloy) is solution treated at 995 – 970 C for about one hour followed by water quenching and then aged at 480 – 590 C for 4 – 8 hours.

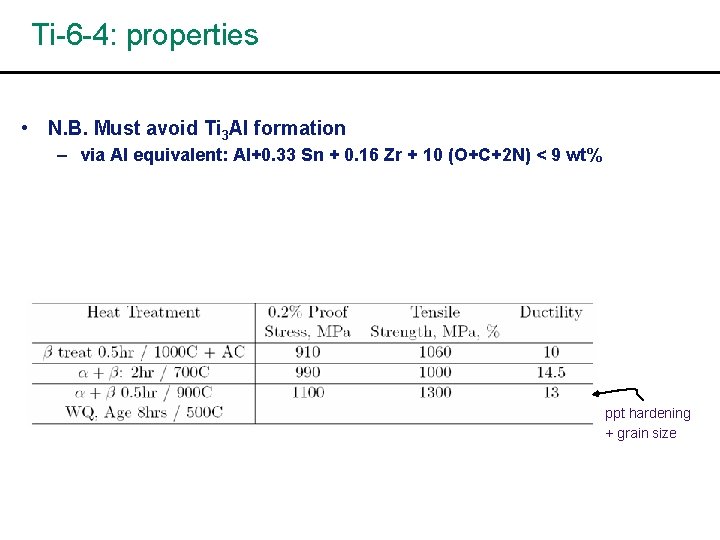
Ti-6 -4: properties • N. B. Must avoid Ti 3 Al formation – via Al equivalent: Al+0. 33 Sn + 0. 16 Zr + 10 (O+C+2 N) < 9 wt% ppt hardening + grain size

Defects • Major α-related problem is the production of α-rich regions due to oxygen (+N) embrittlement – the entrapment of O-rich particles during melting • Also a problem in welding – often Ti is welded in an Ar-filled cavity to avoid this • β alloys suffer from β-rich regions from solute segregation (β flecks), and/or from embrittling ω phase, a diffusionless way to transform from β-bcc to a hexagonal phase.

Burn-resistant β-alloys Titanium fires can occasionally occur in aeroengines or in titanium-based heat exchangers used in the chemical industries. The addition of chromium in concentrations exceeding 10 wt% helps improve the burn-resistance of titanium alloys. The alloy Ti-35 V-15 Cr wt%, has sufficient chromium to resist burning in an aeroengine environment to temperatures up to about 510 o. C. The chromium is not found to be effective in binary Ti-Cr alloys where it does not encourage the formation of a continuous film of protective oxide.

β→ω Transformation • The β→ω transformation is reversible and diffusionless but is not martensitic in the classical sense since there is no invariantplane strain shape deformation. However, it does involve the coordinated motion of atoms. • ω is a metastable phase which forms from β in alloys based on titanium, zirconium. • It is important because its formation generally leads to a deterioration in the mechanical properties. In Ti-Nb alloys its formation influences superconduction. There is also an increase in the electrical resistance as ω forms. • The transformation to ω is diffusion less and frequently cannot be suppressed. Its presence causes diffuse streaking in the electron diffraction patterns of the β phase. The streaks become more intense and curved as the temperature or the solute concentration increases.

β→ω Transformation The body-centred cubic (bcc) crystal structure of β can be imagined as the stacking of {111}β planes in an. . ABCABC. . stacking sequence. Note that these planes are not close-packed in the bcc structure. The β→ω transformation occurs by the passage of a longitudinal displacement wave along <111> which causes the B and C planes to collapse into each other, leaving the A planes unaffected. The stacking sequence thus changes to. . . AB'AB'AB'. . in which the B' planes have twice the density of atoms as the A planes. The. . . AB'AB'AB'. . stacking is consistent with a ω a hexagonal crystal structure with a c/a of about 0. 6. The atoms in the B' plane have a trigonal coordination which is similar to that in graphite and the bonding becomes partly covalent. This leads to an increase in the electrical resistivity. The longitudinal displacement waves are responsible for the streaking in the electron diffraction patterns.

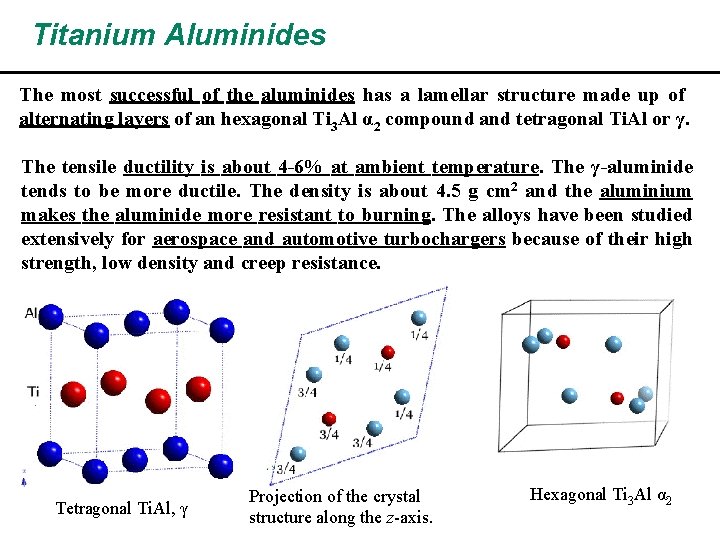
Titanium Aluminides The most successful of the aluminides has a lamellar structure made up of alternating layers of an hexagonal Ti 3 Al α 2 compound and tetragonal Ti. Al or γ. The tensile ductility is about 4 -6% at ambient temperature. The γ-aluminide tends to be more ductile. The density is about 4. 5 g cm 2 and the aluminium makes the aluminide more resistant to burning. The alloys have been studied extensively for aerospace and automotive turbochargers because of their high strength, low density and creep resistance. Tetragonal Ti. Al, γ Projection of the crystal structure along the z-axis. Hexagonal Ti 3 Al α 2

Ti-48 Al at. %: lamellar microstructure of alternating layers of α 2 and γ (Kim and Maruyama, 2001)

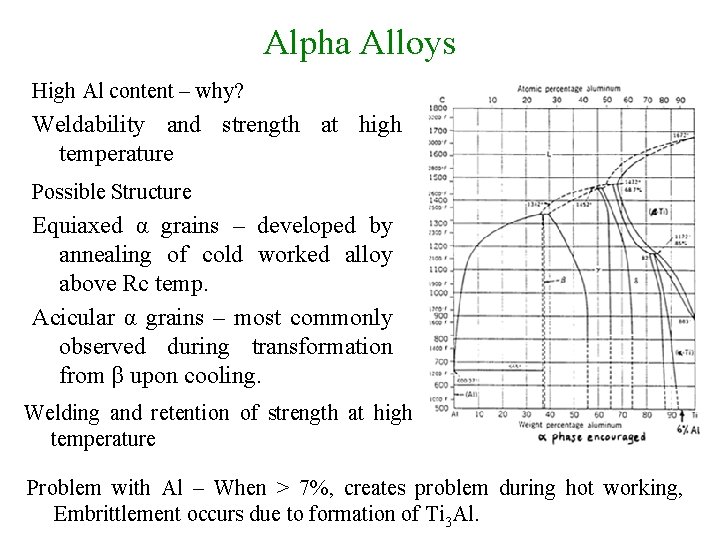
Alpha Alloys High Al content – why? Weldability and strength at high temperature Possible Structure Equiaxed α grains – developed by annealing of cold worked alloy above Rc temp. Acicular α grains – most commonly observed during transformation from β upon cooling. Welding and retention of strength at high temperature Problem with Al – When > 7%, creates problem during hot working, Embrittlement occurs due to formation of Ti 3 Al.

Alpha-Beta alloys Sufficient beta stabilizer. Strengthening Heat treatment - ageing – not conventional – why? ?

Beta alloys Strengthening

Heat Treatments Stress Relieving Annealing Solution Treatment and Ageing

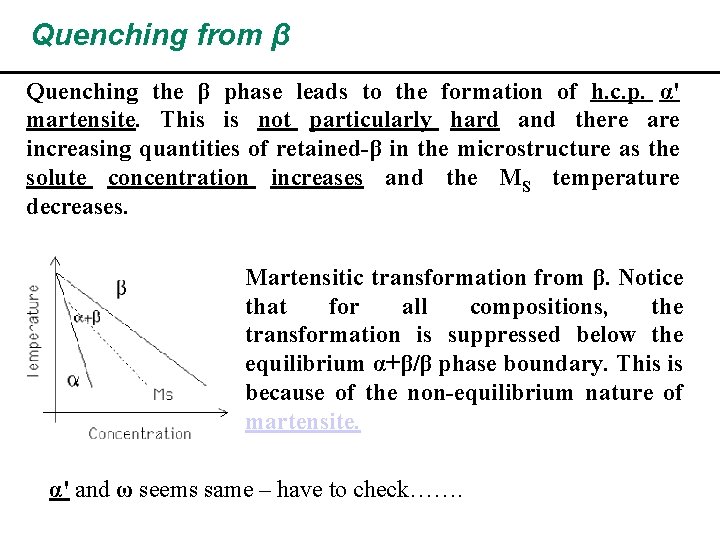
Quenching from β Quenching the β phase leads to the formation of h. c. p. α' martensite. This is not particularly hard and there are increasing quantities of retained-β in the microstructure as the solute concentration increases and the MS temperature decreases. Martensitic transformation from β. Notice that for all compositions, the transformation is suppressed below the equilibrium α+β/β phase boundary. This is because of the non-equilibrium nature of martensite. α' and ω seems same – have to check…….

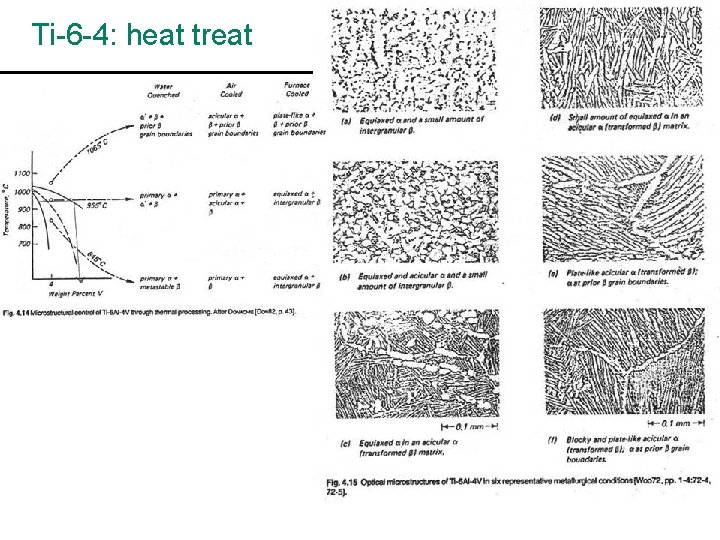
Ti-6 -4: heat treat