INTRODUCTION The various processes used for the actual

























- Slides: 31


INTRODUCTION • The various processes used for the actual recovery of useful products from fermentation or any other process together constitute ‘downstream processing’ • Mainly employed for isolation and purification of biomolecules It Can be divided into following stages: 1)Separation of particles 2)Disintegration of cells 3)Extraction 4)Concentration 5)Purification 6)Drying •

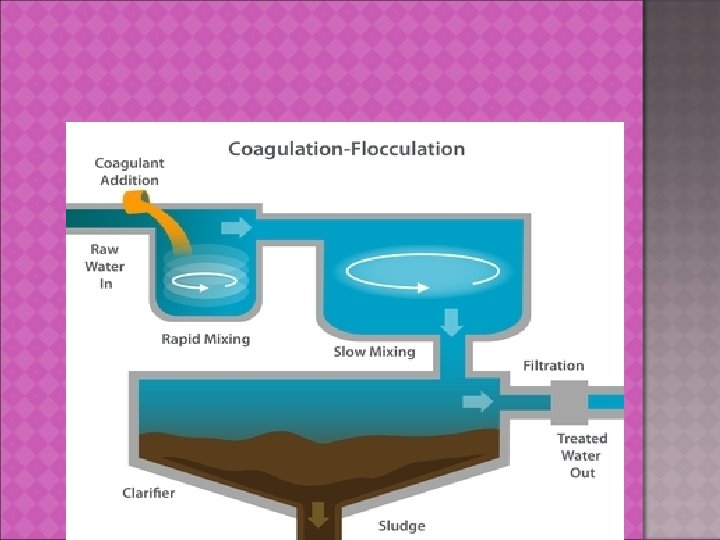
STEP 1: - SEPARATION OF PARTICLES Primary recovery operation Separate whole cells from culture broth, removal of cell debris , collection of protein ppt. etc. Includes a)Filtration b)Centrifugation c)Flocculation d)Floatation

1. 1): FILTERATION A mechanical operation used for the separation of solids from fluids (liquids or gases) by interposing a medium to fluid flow through which the fluid can pass, but the solids in the fluid are retained. Used for separation of filamentous fungi and filamentous bacteria eg. streptomycetes and often yeast flocs. Uses pressure created by overpressure or vacuum. Rate depends on: filter area, fluid viscosity and resistance generated by filter cake

Ø Ø Commonly used: Rotary drum filters Rotating drum with partial vacuum Drum rotates through vacuum Cells form coating on drum Cells scraped off to prevent blocking ROTARY DRUM VACUUM FILTERS

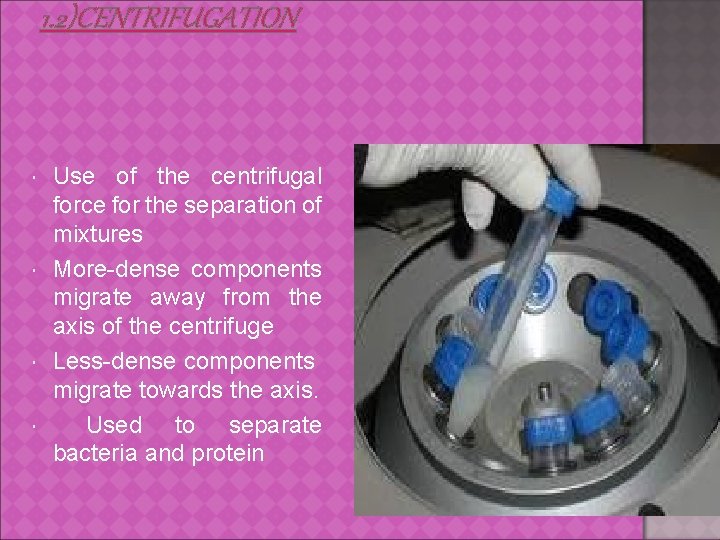
1. 2)CENTRIFUGATION Use of the centrifugal force for the separation of mixtures More-dense components migrate away from the axis of the centrifuge Less-dense components migrate towards the axis. Used to separate bacteria and protein

1. 3) FLOCCULATION AND FLOATATION Used when bacterial cells are not separated by centrifugation Flocculation –sticking together of cells Induced by: - inorganic salts and organic Poly electrolytes Floatation – used where flocculation is not effective


FLOATATION It involves introduction of fine bubbles Fine gas bubbles are created by: Ø Sparger Ø Release of over pressure Ø Electrolysis Gas bubbles adsorb to and surround the cells, raising them to surface of medium in form of foam (floatation) Fatty acids and amines promote stable foam formation

STEP 2: - DISINTEGRATION OF CELLS Disruption of microbial cells is difficult due to: Ø Small size Ø Strong cell wall Ø High osmotic pressure It is achieved by : Ø Mechanical means Ø Lysis Ø Drying

2. 1)MECHANICAL CELL DISRUPTION Uses shear , pressure and pressure release Shear –grinding in ball mill --colloid mill Pressure –homogenizer -ultrasound Cell suspension is forced through fine nozzle Cells disintegrate due to shear

2. 2) DRYING Widely used method Dried by adding cells into large excess of cold acetone Followed by extraction Extracted using buffer or salt solution Induces changes in cell wall structure which facilitate extraction

2. 3) LYSIS Lysis can be achieved by two means: a)chemical means b)lytic enzymes Chemical means: -salts, surfactants, osmotic shock, freezing Lytic enzymes: -lysozymes

STEP 3): - EXTRACTION Process of recovering a compound or group of compound from a mixture or from cells into a solvent phase is called extraction Involves both separation as well as concentration of product Useful for recovery of lipophilic substances and in antibiotic recovery Early step after cell separation

3. 1)LIQUID -LIQUID EXTRACTION Employs two immiscible liquids into which product is differentially soluble Smaller volumes of solvent are used for repeated extraction of a given sample Back extraction tends to increase sensitivity of extraction

STEP 4): -CONCENTRATION Some concentration occur during extraction process Final concentration may be achieved by: Ø Evaporation Ø Membrane filteration Ø Ion exchange methods Ø Adsorption methods

4. 1): -EVAPORATION Used in case of solvent extraction Efficient arrangements are made for recovery of evaporated solvent to reduce cost For low grade products evaporation of whole broth is done using spray drier Spray drying Requires use of heat to evaporate water – unsuitable for most proteins

4. 2)MEMBRANE FILTERATION Generally achieves both concentration and separation of the products on the basis of size It includes: - microfiltration , ultra filtration , reverse osmosis and electro dialysis Micro and ultra filtration separates molecules of diff sizes Reverse osmosis $ electro dialysis separate molecules of same size

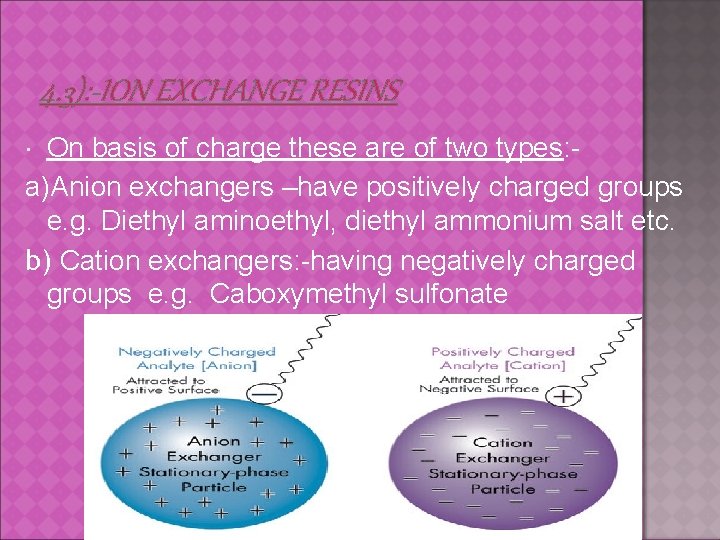
4. 3): -ION EXCHANGE RESINS On basis of charge these are of two types: a)Anion exchangers –have positively charged groups e. g. Diethyl aminoethyl, diethyl ammonium salt etc. b) Cation exchangers: -having negatively charged groups e. g. Caboxymethyl sulfonate

4. 4): -ADSORPTION RESINS Porous polymers Without ionization Compounds adsorb to resin in non ionised state Porosity determines surface availability for adsorption Resins may be : a) Apolar – e. g. Styrene divinyl benzene b)Polar : e. g. Sulphoxide , amide etc. c)Semipolar : - eg. Acrylic esters The product from such resins are recovered by solvent extraction , changed p. H etc.

STEP 5): - PURIFICATION Aims at obtaining product in highly purified state It is achieved by: Ø Crystallisation Ø Chromatographic procedures

5. 1)CRYSTALLIZATION Process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Chemical solid-liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. Mainly used for purification of low molecular weight compounds eg. In case of antibiotics like penicillin G Final stage in purification of products

5. 2): -CHROMATOGRAPHIC METHODS Used for purification of low molecular wt. compounds from mixture of similar molecules It includes: a)Adsorption chromatography b)Ion exchange chromatography c)Gel permeation chromatography d)Affinity chromatography Separates molecules due to their differential affinities for the surface of solid matrix Eg. Of solid matrix are- silica gel, hydroxyapetite or an organic polymer

v Ion exchange chromatography Involves binding and separation of proteins based on charge-charge interactions • Proteins bind at low ionic strength, and are eluted at high ionic strength •

v. AFFINITY CHROMATOGRAPHY Use molecules called effectors Product has high and specific affinity for effectors Effector is immobilised on water insoluble carrier Carrier is packed in column through which mixture is passed Effector binds to only those molecules for which it is specific and retains in the column Later recovered by elution using buffer solution of specific p. H

v. GEL PERMEATION CHROMATOGRAPHY (GPC) Also known as ‘size exclusion chromatography’ and ‘gel filtration chromatography’ Separates molecules on the basis of molecular size Separation is based on the use of a porous matrix. Small molecules penetrate into the matrix more, and their path length of elution is longer. Large molecules appear first, smaller molecules later

STEP 6): - DRYING Last step in DSP Makes product suitable for handling and storage Should be accomplished with minimum rise in temperature It involves a)Vaccum drying b)Spray drying c)Freeze drying

6. 1): -SPRAY DRYING Solution or slurry to be dried is atomized by nozzle or rotating disc Hot air current is passed (150 -250. c) Drying is so rapid that temp. of particles remain low Used for enzymes , food products and antibiotics

6. 2): - VACUUM DRYING Uses both heat and vacuum Can be applied both in batch mode and continuous mode More effective

6. 3): - FREEZE DRYING Liquor to be dried is first frozen Water is sublimed from frozen mass Low pressure is maintained to promote sublimation Energy for sublimation is provided by heated plates and radiation onto the surface Temperature is regulated by regulating pressure in drying chamber Most gentle method of drying Used mainly out of all Used for many pharmaceutical products like vaccines , enzymes etc.

THANK YOU