Introduction Teaching Points a Symbols b Some Metals

Introduction • Teaching Points: (a) Symbols • (b) Some Metals and Non-Metals and their Symbols • Specific Aim : To appreciate the idea of Symbol and its significance, to become familiar with symbols of some Metals and Non-Metals.

Some Questions • • • Question: Do we have our individual names? Answer: Yes Question: What is the requirement of names? Answer: Individual names are required to call someone and at the same time the individual also responds. Question: What is the requirement to name all elements found on Earth? Answer: To give names to all Elements found on Earth is to identify them individually across the world.

Some Questions • • • Question: What is the requirement of Pet names? Answer: Pet name is a short name. Sometimes the actual name appears too long! Question: Can we have pet name for each and every Element? Answer: Yes. Question: What way would you like to have the Pet names for Elements? Answer: With the Help of Symbol….

Introduction • You know cadets; • Element names in most cases are too long and difficult to memories. • Elements are therefore given Pet names known as Symbols. • Today, we shall discuss about Symbols, Symbols of some Metals and Non-Metals.

Symbol: o The short form used to refer to an element is called a Symbol. Rules: Each Element is represented by the first letter of its name written in capitals. e. g. Hydrogen is written as H, Oxygen is written as O etc. If two elements have names beginning with the same letter, then a second letter in lowercase is written with the first letter of the name. e. g. Calcium is written as Ca, Chlorine is written as Cl etc.
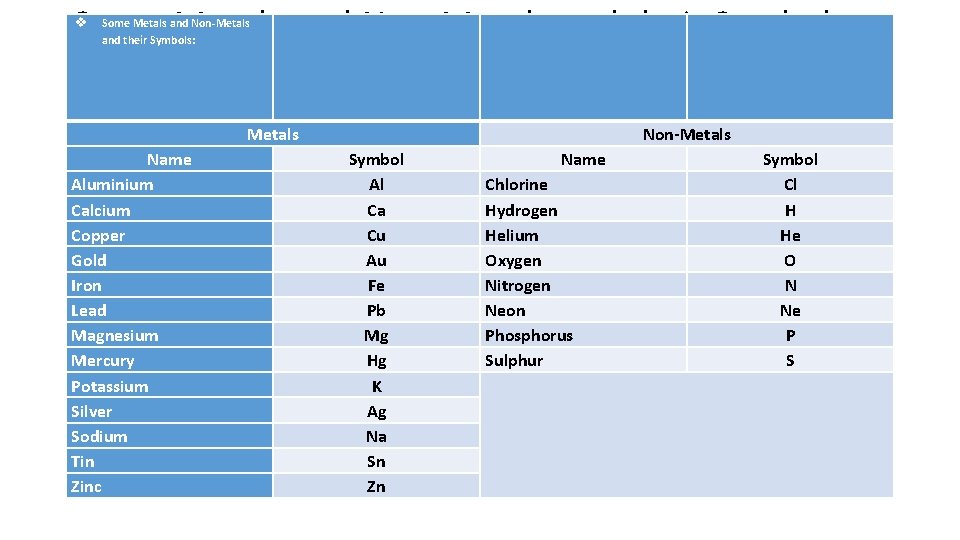
Some Metals and Non-Metals and their Symbols: Metals Name Aluminium Calcium Copper Gold Iron Lead Magnesium Mercury Potassium Silver Sodium Tin Zinc Non-Metals Symbol Al Ca Cu Au Fe Pb Mg Hg K Ag Na Sn Zn Name Chlorine Hydrogen Helium Oxygen Nitrogen Neon Phosphorus Sulphur Symbol Cl H He O N Ne P S

Some Questions & Answers • • • Question: Give the definition of Symbol. Answer: The short form used to refer to an element is called a Symbol. Question: How the symbol for an Element is written? Answer: Each Element is represented by the first letter of its name written in capitals. e. g. Hydrogen is written as H, Oxygen is written as O etc. If two elements have names beginning with the same letter, then a second letter in lowercase is written with the first letter of the name. e. g. Calcium is written as Ca, Chlorine is written as Cl etc.

Some Questions & Answers • • • Question: Give the Symbols of Aluminium, Gold, Mercury, Sodium, Zinc. Answer: (Student) Aluminium-Al, Gold-Au, Mercury-Hg, Sodium- Na, Zinc-Zn. Question: Give the Symbols of Chlorine, Helium, Neon, Phosphorus, Sulphur. Answer: Chlorine-Cl, Helium-He, Neon-Ne, Phosphorus-P, Sulphur-S. Question: Can the Symbol for an Element vary from place to place? Answer: No, the Symbol for an Element remains the same worldwide. We cannot change it.

Question? • Question: • Mention the advantages of having symbols. • Answer: • Element names in most cases are too long and difficult to memorise. • Symbols are small and easy to remember. • The Symbol for an Element remains the same worldwide. We cannot change it.
- Slides: 9