Introduction Synthesis of PDT Compound In recent years
- Slides: 1

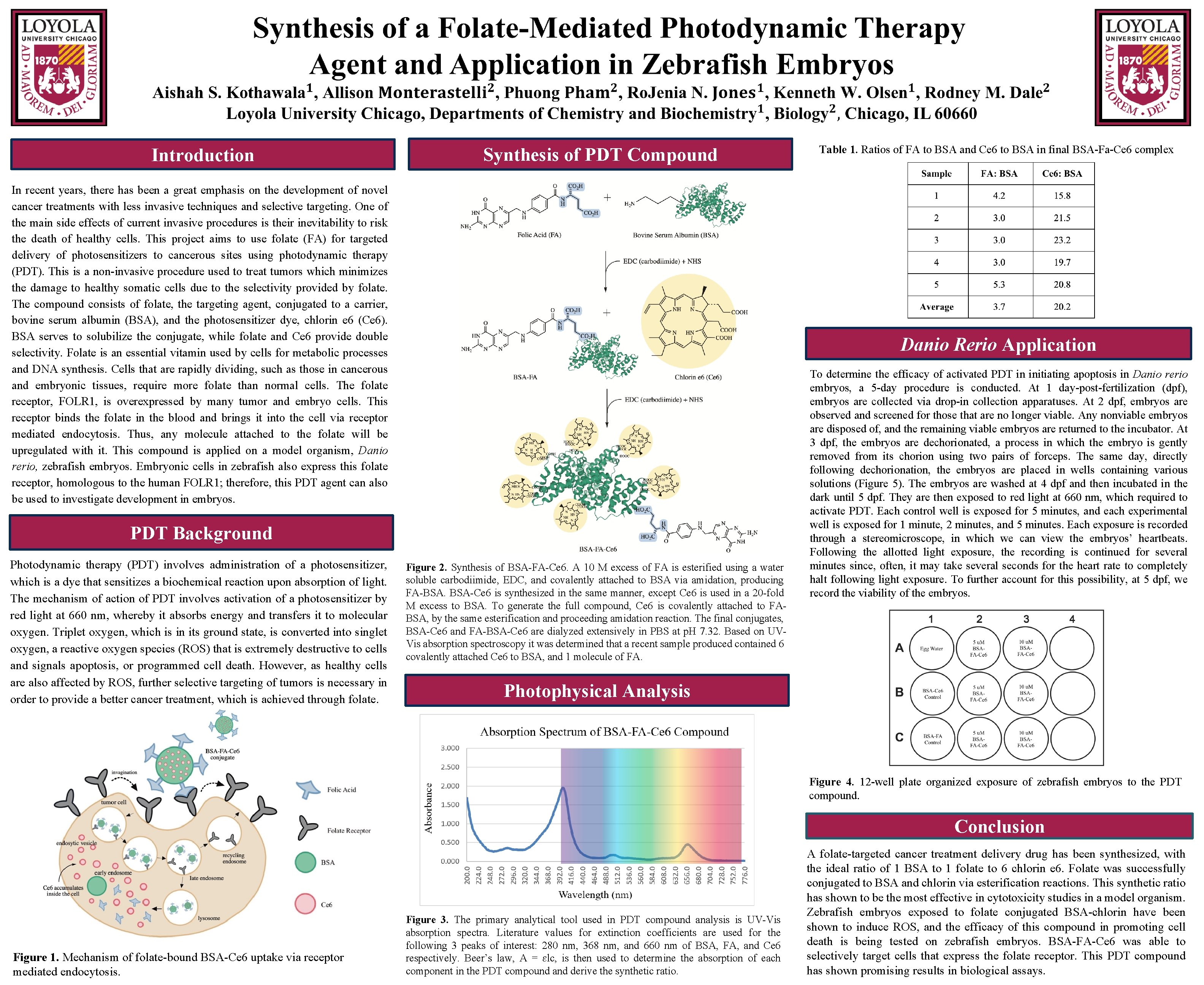
Introduction Synthesis of PDT Compound In recent years, there has been a great emphasis on the development of novel cancer treatments with less invasive techniques and selective targeting. One of the main side effects of current invasive procedures is their inevitability to risk the death of healthy cells. This project aims to use folate (FA) for targeted delivery of photosensitizers to cancerous sites using photodynamic therapy (PDT). This is a non-invasive procedure used to treat tumors which minimizes the damage to healthy somatic cells due to the selectivity provided by folate. The compound consists of folate, the targeting agent, conjugated to a carrier, bovine serum albumin (BSA), and the photosensitizer dye, chlorin e 6 (Ce 6). BSA serves to solubilize the conjugate, while folate and Ce 6 provide double selectivity. Folate is an essential vitamin used by cells for metabolic processes and DNA synthesis. Cells that are rapidly dividing, such as those in cancerous and embryonic tissues, require more folate than normal cells. The folate receptor, FOLR 1, is overexpressed by many tumor and embryo cells. This receptor binds the folate in the blood and brings it into the cell via receptor mediated endocytosis. Thus, any molecule attached to the folate will be upregulated with it. This compound is applied on a model organism, Danio rerio, zebrafish embryos. Embryonic cells in zebrafish also express this folate receptor, homologous to the human FOLR 1; therefore, this PDT agent can also be used to investigate development in embryos. Danio Rerio Application PDT Background Photodynamic therapy (PDT) involves administration of a photosensitizer, which is a dye that sensitizes a biochemical reaction upon absorption of light. The mechanism of action of PDT involves activation of a photosensitizer by red light at 660 nm, whereby it absorbs energy and transfers it to molecular oxygen. Triplet oxygen, which is in its ground state, is converted into singlet oxygen, a reactive oxygen species (ROS) that is extremely destructive to cells and signals apoptosis, or programmed cell death. However, as healthy cells are also affected by ROS, further selective targeting of tumors is necessary in order to provide a better cancer treatment, which is achieved through folate. Table 1. Ratios of FA to BSA and Ce 6 to BSA in final BSA-Fa-Ce 6 complex Figure 2. Synthesis of BSA-FA-Ce 6. A 10 M excess of FA is esterified using a water soluble carbodiimide, EDC, and covalently attached to BSA via amidation, producing FA-BSA. BSA-Ce 6 is synthesized in the same manner, except Ce 6 is used in a 20 -fold M excess to BSA. To generate the full compound, Ce 6 is covalently attached to FABSA, by the same esterification and proceeding amidation reaction. The final conjugates, BSA-Ce 6 and FA-BSA-Ce 6 are dialyzed extensively in PBS at p. H 7. 32. Based on UVVis absorption spectroscopy it was determined that a recent sample produced contained 6 covalently attached Ce 6 to BSA, and 1 molecule of FA. To determine the efficacy of activated PDT in initiating apoptosis in Danio rerio embryos, a 5 -day procedure is conducted. At 1 day-post-fertilization (dpf), embryos are collected via drop-in collection apparatuses. At 2 dpf, embryos are observed and screened for those that are no longer viable. Any nonviable embryos are disposed of, and the remaining viable embryos are returned to the incubator. At 3 dpf, the embryos are dechorionated, a process in which the embryo is gently removed from its chorion using two pairs of forceps. The same day, directly following dechorionation, the embryos are placed in wells containing various solutions (Figure 5). The embryos are washed at 4 dpf and then incubated in the dark until 5 dpf. They are then exposed to red light at 660 nm, which required to activate PDT. Each control well is exposed for 5 minutes, and each experimental well is exposed for 1 minute, 2 minutes, and 5 minutes. Each exposure is recorded through a stereomicroscope, in which we can view the embryos’ heartbeats. Following the allotted light exposure, the recording is continued for several minutes since, often, it may take several seconds for the heart rate to completely halt following light exposure. To further account for this possibility, at 5 dpf, we record the viability of the embryos. Photophysical Analysis Figure 4. 12 -well plate organized exposure of zebrafish embryos to the PDT compound. Conclusion Figure 1. Mechanism of folate-bound BSA-Ce 6 uptake via receptor mediated endocytosis. Figure 3. The primary analytical tool used in PDT compound analysis is UV-Vis absorption spectra. Literature values for extinction coefficients are used for the following 3 peaks of interest: 280 nm, 368 nm, and 660 nm of BSA, FA, and Ce 6 respectively. Beer’s law, A = ɛlc, is then used to determine the absorption of each component in the PDT compound and derive the synthetic ratio. A folate-targeted cancer treatment delivery drug has been synthesized, with the ideal ratio of 1 BSA to 1 folate to 6 chlorin e 6. Folate was successfully conjugated to BSA and chlorin via esterification reactions. This synthetic ratio has shown to be the most effective in cytotoxicity studies in a model organism. Zebrafish embryos exposed to folate conjugated BSA-chlorin have been shown to induce ROS, and the efficacy of this compound in promoting cell death is being tested on zebrafish embryos. BSA-FA-Ce 6 was able to selectively target cells that express the folate receptor. This PDT compound has shown promising results in biological assays.