Introduction Reaction Rate Collision Theory Mr Shields Regents




- Slides: 16

Introduction, Reaction Rate & Collision Theory Mr. Shields Regents Chemistry U 13 L 04 1

Introduction To Kinetics In the Past we’ve discussed such concepts as - Kinetic Energy - Kinetic Molecular Theory So … what does the word “KINETIC” mean? - Movement, being in a state of motion 2

Introduction To Kinetics So … Kinetic Energy is the Energy of Motion - What is Avg. KE a measure of? - Temperature It can also be used to determine Molecular Velocity - KE = ½ mv 2 And Kinetic Molecular Theory as we’ve seen attempts to Explain the behavior of ideal Gases in response to Changes in P, T or V. 3

Chemical Kinetics attempts to describe how quickly a Chemical Reaction will occur For instance does reaction 1 and reaction 2 take place At the same or different rates? 1) N 2 + 3 H 2 2 NH 3 2) Pb. NO 3 + KI Pb. I + KNO 3 KI Will these rxns be instantaneous or do they take place over several minutes, several hours or even days? Answering this Question involves understanding the kinetics of the reaction! 4

Chemical Kinetics Consider for example some ground beef - Cook up a few hamburgers and Leave them in a cold frig … and they may last a week - leave them on the counter in a warm kitchen and they may go bad in a day - Both hamburgers (in the frig and on the counter) are decaying over time (a chemical reaction) but 5 these chemical rxns occur at DIFFERENT RATES

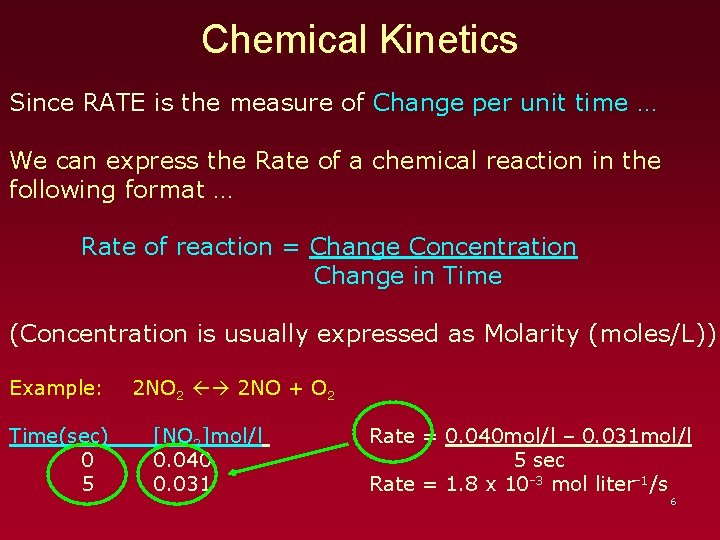
Chemical Kinetics Since RATE is the measure of Change per unit time … We can express the Rate of a chemical reaction in the following format … Rate of reaction = Change Concentration Change in Time (Concentration is usually expressed as Molarity (moles/L)) Example: Time(sec) 0 5 2 NO 2 2 NO + O 2 [NO 2]mol/l 0. 040 0. 031 Rate = 0. 040 mol/l – 0. 031 mol/l 5 sec Rate = 1. 8 x 10 -3 mol liter-1/s 6

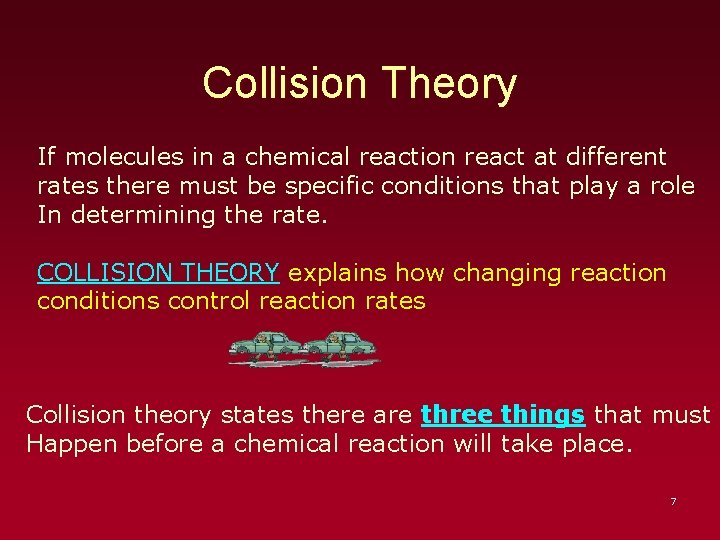
Collision Theory If molecules in a chemical reaction react at different rates there must be specific conditions that play a role In determining the rate. COLLISION THEORY explains how changing reaction conditions control reaction rates Collision theory states there are three things that must Happen before a chemical reaction will take place. 7

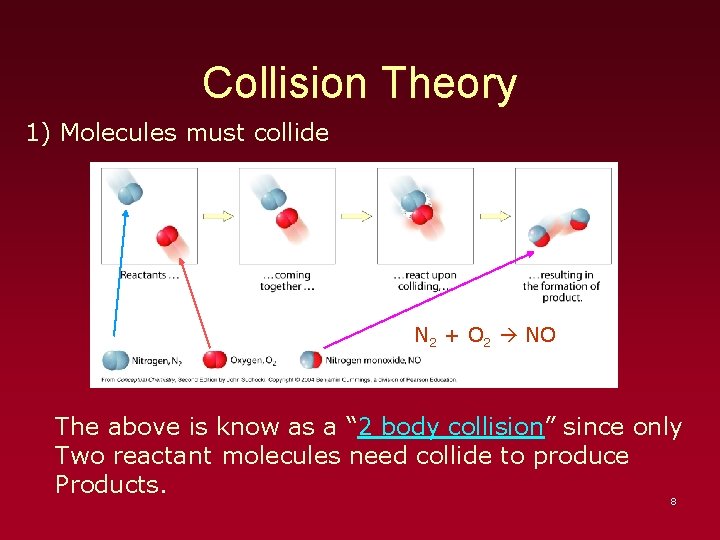
Collision Theory 1) Molecules must collide N 2 + O 2 NO The above is know as a “ 2 body collision” since only Two reactant molecules need collide to produce Products. 8

Collision Theory states that MOST chemical reactions Involve 2 body collisions So… If most reactions are only two body collisons then How do we explain reactions with more than 2 Reactants? Nitrogen oxyfluoride For example: 2 NO(g) + F 2 2 NOF(g) i. e. NO + F 2 NOF + NOF According to this eqn, 2 nitrogen (II) oxide molecules and a fluorine molecule must collide at the same time to yield 2 NOF. But this looks like a 3 body collision, right? 9

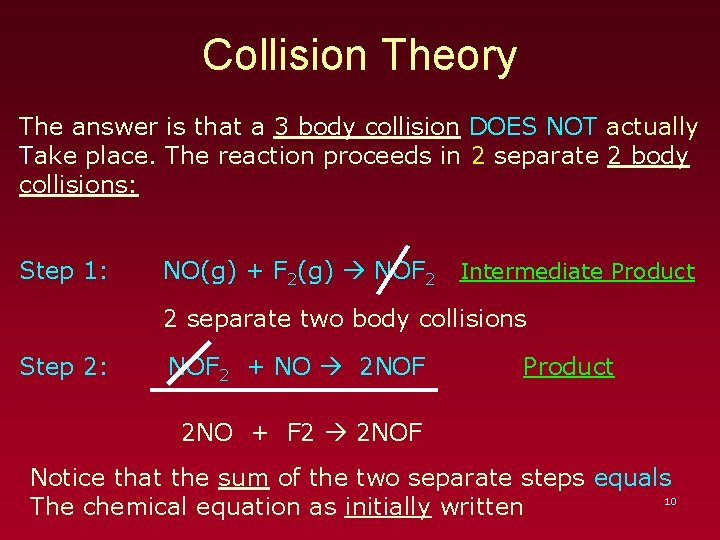
Collision Theory The answer is that a 3 body collision DOES NOT actually Take place. The reaction proceeds in 2 separate 2 body collisions: Step 1: NO(g) + F 2(g) NOF 2 Intermediate Product 2 separate two body collisions Step 2: NOF 2 + NO 2 NOF Product 2 NO + F 2 2 NOF Notice that the sum of the two separate steps equals 10 The chemical equation as initially written

Orientation In Collison theory we said there were three factors Necessary for a chemical reaction to take place. The first factor, as we’ve seen is that Molecules Must Collide. The 2 nd factor is: During collision the Molecules Must be in the Proper Orientation OK. So what does PROPER ORIENTATION mean? 11

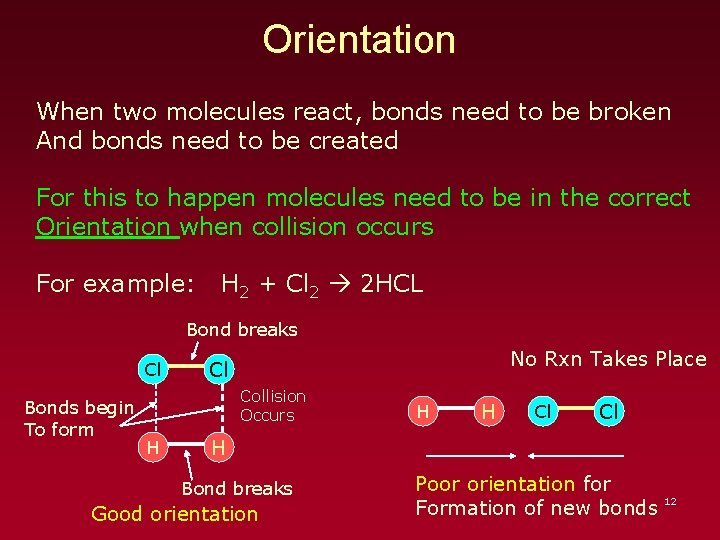
Orientation When two molecules react, bonds need to be broken And bonds need to be created For this to happen molecules need to be in the correct Orientation when collision occurs For example: H 2 + Cl 2 2 HCL Bond breaks Cl Bonds begin To form No Rxn Takes Place Cl Collision Occurs H H H Cl Cl H Bond breaks Good orientation Poor orientation for 12 Formation of new bonds

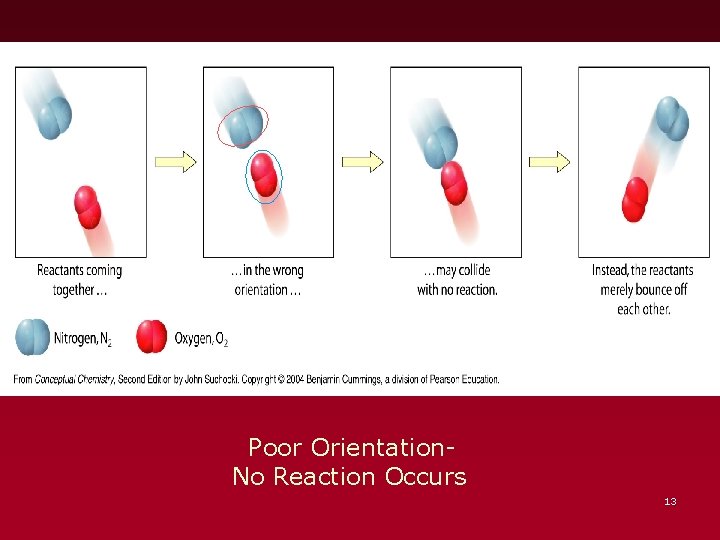
Poor Orientation. No Reaction Occurs 13

Kinetic Energy So to reiterate, collision theory says that for molecules To react they must: 1) Collide 2) Be in the right orientation at collision And lastly besides these two requirements, molecules Must have … 3) Sufficient kinetic energy when they collide for the reaction to occur – in other words they must collide 14 with sufficient velocity

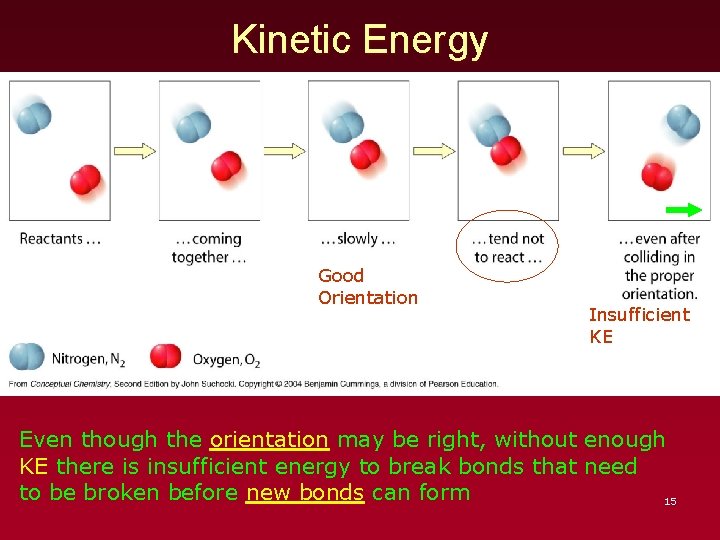
Kinetic Energy Good Orientation Insufficient KE Even though the orientation may be right, without enough KE there is insufficient energy to break bonds that need to be broken before new bonds can form 15

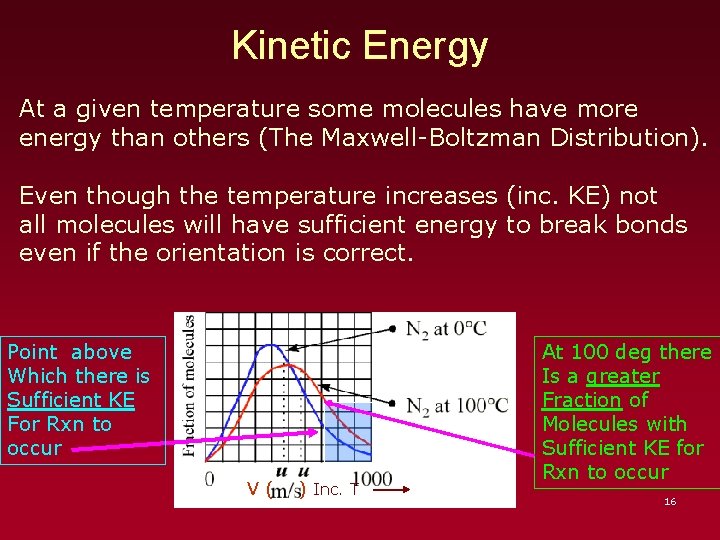
Kinetic Energy At a given temperature some molecules have more energy than others (The Maxwell-Boltzman Distribution). Even though the temperature increases (inc. KE) not all molecules will have sufficient energy to break bonds even if the orientation is correct. Point above Which there is Sufficient KE For Rxn to occur V( ) Inc. T At 100 deg there Is a greater Fraction of Molecules with Sufficient KE for Rxn to occur 16