Introduction Plastics 001 Plastics Introduction to Materials www





















- Slides: 24

Introduction Plastics 001 Plastics Introduction to Materials www. recyclingghk. com

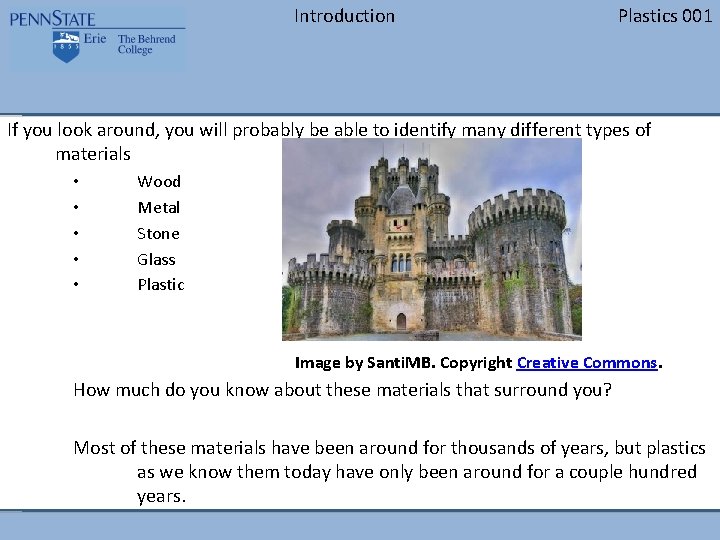
Introduction Plastics 001 If you look around, you will probably be able to identify many different types of materials • • • Wood Metal Stone Glass Plastic Image by Santi. MB. Copyright Creative Commons. How much do you know about these materials that surround you? Most of these materials have been around for thousands of years, but plastics as we know them today have only been around for a couple hundred years.

Introduction Why do we use plastic? 1. Versatile • Can easily be formed into very complex shapes 2. Relatively Inexpensive 3. Many processes are easily automated 4. High strength to weight ratio 5. Can quickly produce a lot of parts Plastics 001

Introduction Why do we use plastic? 6. Good insulation properties • • Thermal Electrical 7. Corrosion resistance 8. Can be made transparent or easily colored 9. Capable of being foamed • • Lightweight Flexible Many of the products used today would not be possible without plastics. Plastics 001

Introduction Plastics 001 When you hear the word PLASTIC, what comes to mind? What is a ‘PLASTIC’? What is a ‘POLYMER’? How long have plastics been around? How much plastic is used each year?

Introduction Plastics 001 When you hear the word PLASTIC, what comes to mind? According to Dictionary. com a plastic can be defined as Any of a group of synthetic or natural organic materials that may be shaped when soft and then hardened, including many types of resins, resinoids, polymers, cellulose derivatives, casein materials, and proteins: used in place of other materials, as glass, wood, and metals, in construction and decoration, for making many articles, as coatings, and, drawn into filaments, for weaving. They are often known by trademark names, as Bakelite, Vinylite, or Lucite. http: //dictionary. reference. com/browse/plastic

Introduction Plastics 001 The definition of a polymer is: Polymer - Any of numerous natural and synthetic compounds of usually high molecular weight consisting of up to millions of repeated linked units, each a relatively light and simple molecule. Most of the materials we refer to as plastics are technically polymers. http: //dictionary. reference. com/browse/polymers

Introduction Plastics 001 Basically a plastic is a material that you can shape when it is soft and it will then harden. It can be a naturally occurring material like clay. It can be a synthetic material like Polyethylene.

Introduction Plastics 001 When we think of plastics today, we are primarily talking about polymers or a group of synthetic organic compounds, that are heated and shaped to make items ranging from children’s toys to automotive components to high tolerance medical parts. An organic compound is a material that is composed of primarily Carbon (C). Today’s plastics are mostly created from oil and natural gas – but some are created from agricultural sources like corn.

Introduction Plastics 001 The word POLYMER comes from the Greek language and basically means many ‘mers’ – – – A ‘mer’ is a unit Polyethylene means many ‘ethylenes’ Think of it like each unit is a bead and the final molecule is the string of beads – only the string usually has several thousand beads Ethylene Polyethylene H H | | C= C | | H HHHHHHH | | | | [-C-C-C-C-C-C-C-] | | | | H HHHHHHH

Introduction Plastics 001 Molecular Weight The molecular weight of a polymer depends on the length of the chain (The number of ‘mers’ (beads) times the weight of the ‘mer’) The length of the chain will effect the properties or the molecule and the state or phase of the material • 2 ethylene ‘mers’ = butane (a gas found in lighters) • 4 ethylene ‘mers’ = octane (gasoline) • 10 -20 ethylene ‘mers’ = greases or oils • 200 -300 = waxes • 10, 000 + = polyethylene Each polyethylene ‘mer’ has a molecular A mole is a unit of measure weight of 28 grams/mole, so a molecular for atoms and molecules chain of polyethylene 10, 000 ‘mers’ long would have a molecular weight of 280, 000 grams/mole

Introduction Plastics 001 Structure The structure of polymer materials leads to their unique properties as well as some of their disadvantages. –Dimensional instability –Some absorb moisture –Many are flammable –Some are attacked or dissolved by certain chemicals –Many take a long time to degrade when disposed of Different material families will have different disadvantages, which is why it is very important to choose the right material for the right application.

Introduction Plastics 001 History Natural polymers have been around longer than we have. Natural rubbers, cellulose, spider webs, and animal horns are all examples of natural polymers. Man has worked with natural polymers to create objects for a long, long time. It wasn’t until the mid 1800’s that man began to modify natural polymers to make them easier to process and increase their usefulness.

Introduction History Much of the development/discovery of plastics has either been in the attempt to replace other materials or through accidental discovery. – Much of the development of PVC (Vinyl) was in an attempt to replace rubber – Nylon development was spurred on by the need to replace silk in parachutes and ropes – Teflon (Polytetrafluoroethylene) was discovered accidentally in a Du. Pont Lab when working on alternative refrigerants. – Celluloid was developed in response to a need to replace ivory in billiard balls. Plastics 001

Introduction Plastics 001 Volume The ages of man are listed as; Stone Age – man made primitive tools and weapons from stone. Bronze Age – man began to smelt copper and tin to make items Iron Age – man began to work iron The late 20 th century and on could very well be named the Plastic Age.

Introduction Plastics 001 Volume Many of the technological developments of the past 50 years would not have been possible without the discovery and development of plastic materials Plastics have allowed for the miniaturization of many of today’s common devices: • • Laptops Cell phones Ipods Etc.

Introduction Volume • • • Plastics are highly used in almost every industry Automotive Medical Construction Packaging Consumer goods Plastics 001

Introduction Plastics 001 Volume In 2006 there were 113. 2 billion pounds of plastic produced worldwide. Making products out of plastic saves energy not only in the production of the items, but also in the transportation of those items. Because of the incredible volume of plastics used in today’s society, many activist and political groups are concerned with the recyclability of plastic materials.

Introduction Plastics 001 Recycling Plastic materials do not decompose quickly in a landfill environment. – The lack of oxygen and sunlight slows the decomposition process. This is the reasoning behind two main initiatives in the plastics industry 1. Recycling 2. The development of degradable polymers

Introduction Plastics 001 Recycling On average we recycle around 27% of the plastic products that are produced with some materials being as high as 60%

Introduction Recycling Common products made with recycled materials: Carpet Rope Flower Pots Trash Cans Drainage Pipes Grocery Bags Plastic Lumber Plastics 001

Introduction Plastics 001 Volume Biodegradable materials The other option to recycling is the development of degradable materials. The development of these materials is also driven due to the rising cost of oil and natural gas. The most prominent material in this area is probably Polylactic Acid which is processed from corn.

Introduction Plastics 001 Material Families Of the 113. 2 billion pounds of plastic materials produced in 2006; the major material ‘families’ are broken down in the following distribution. Polyethylene 9 Million Metric Tons Polypropylene 8. 4 Million Metric Tons Poly Vinyl Chloride (PVC) 7. 3 Million Metric Tons Polycarbonate 3 Million Metric Tons

Introduction Plastics Introduction Questions? Plastics 001
 Cant stop the feeling go noodle
Cant stop the feeling go noodle Useful and harmful materials images
Useful and harmful materials images Man made materials
Man made materials Adopting materials
Adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Heterojenleşme nedir
Heterojenleşme nedir Critical value of 0.001
Critical value of 0.001 Promille
Promille Acad 02-001
Acad 02-001 Ms 08-067
Ms 08-067 Nom 001 stps 2008 edificios locales e instalaciones
Nom 001 stps 2008 edificios locales e instalaciones Numero decimal
Numero decimal 99366 cpt code
99366 cpt code Mama zosi kupiła 5 długopisów po 8 zł każdy
Mama zosi kupiła 5 długopisów po 8 zł każdy Pyp-001
Pyp-001 Aes 001
Aes 001 011 101 001
011 101 001 Tms320dm
Tms320dm Vnf descriptor
Vnf descriptor Semt.001
Semt.001 Write 702 001 in expanded form
Write 702 001 in expanded form Nf p 01 012
Nf p 01 012 Easa cm-swceh-001
Easa cm-swceh-001 In mudra loan rs.50 001 to rs.500 000 are categorised as
In mudra loan rs.50 001 to rs.500 000 are categorised as Auec2-001
Auec2-001