INTRODUCTION OF A PIPERACILLIN AND TAZOBACTAM PATIENT GROUP

INTRODUCTION OF A PIPERACILLIN AND TAZOBACTAM PATIENT GROUP DIRECTION (PGD) IN HAEMATOLOGY C Mc. Caughey, D Mc. Kelvey, J Stewart, C Mallon, P Scullin

Background • Neutropenic sepsis (NS) is a potentially fatal complication of systemic anti-cancer therapy National Chemotherapy Advisory Group 2009
NS toolkit Regional Policy Staff Education Care Bundles Care Pathway
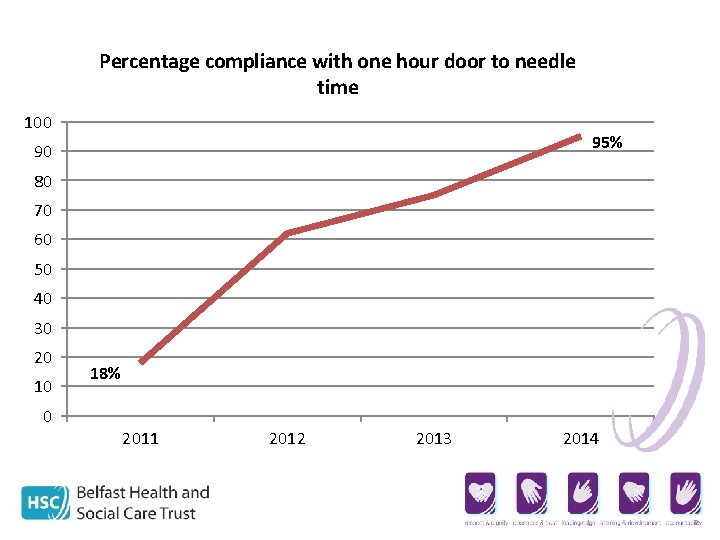
Percentage compliance with one hour door to needle time 100 95% 90 80 70 60 50 40 30 20 10 0 18% 2011 2012 2013 2014

. . but 100% of door to needle breaches out of hours

Patient Group Direction • PGDs provide a legal framework that allows the supply and/or administration of a specified medicine(s), by named, authorised, registered health professionals, to a predefined group of patients needing prophylaxis or treatment for a condition described in the PGD, without the need for a prescription or an instruction from a prescriber. (NICE, 2013)
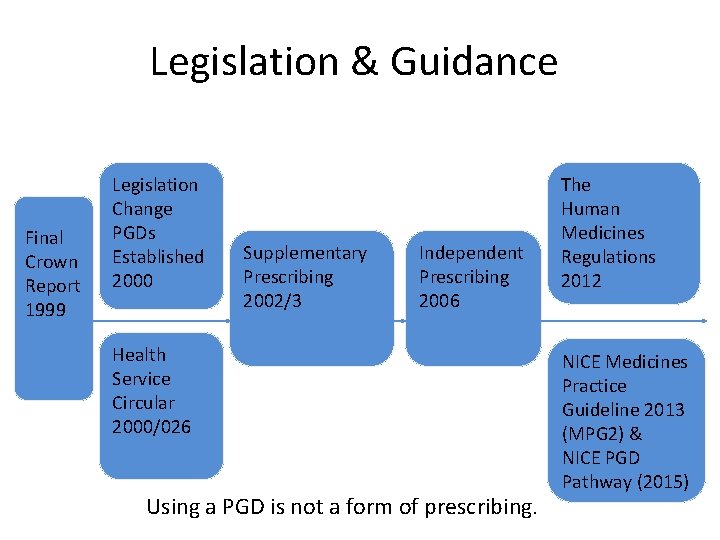
Legislation & Guidance Final Crown Report 1999 Legislation Change PGDs Established 2000 Supplementary Prescribing 2002/3 Independent Prescribing 2006 Health Service Circular 2000/026 Using a PGD is not a form of prescribing. The Human Medicines Regulations 2012 NICE Medicines Practice Guideline 2013 (MPG 2) & NICE PGD Pathway (2015)
PGD proposed, written and authorised Pharmacy Nursing Medicine Microbiology

Criteria for staff utilising PGD Registered nurses who have following training: l Venepuncture and cannulation (one of training) l Administration of medicines and medicines code update (three yearly update) l Anaphylaxis (two yearly update) l Resuscitation (in hospital life support two yearly update) l PGD training
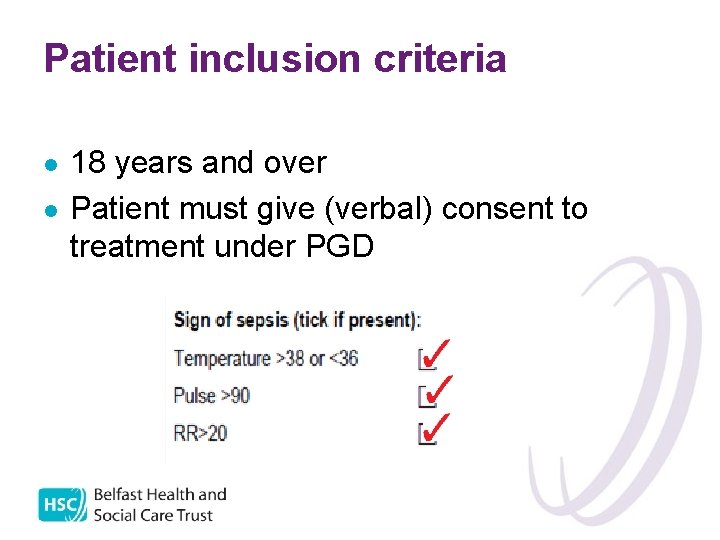
Patient inclusion criteria l l 18 years and over Patient must give (verbal) consent to treatment under PGD
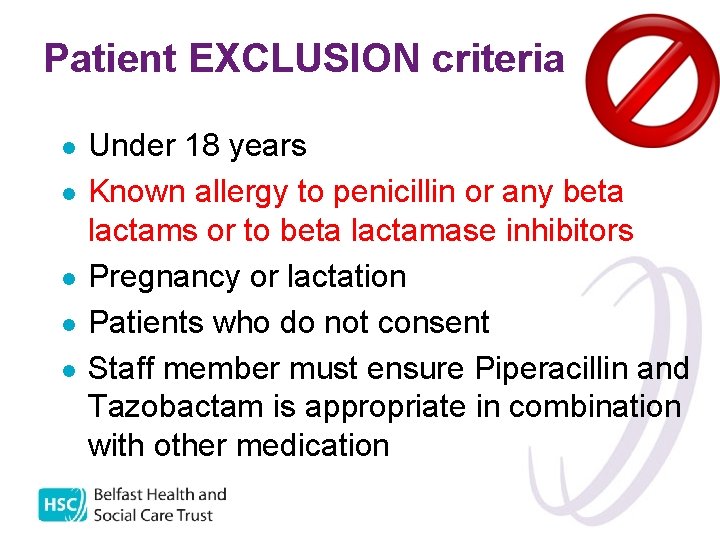
Patient EXCLUSION criteria l l l Under 18 years Known allergy to penicillin or any beta lactams or to beta lactamase inhibitors Pregnancy or lactation Patients who do not consent Staff member must ensure Piperacillin and Tazobactam is appropriate in combination with other medication


Models of training and assessment • • Nursing or pharmacy led face to face sessions Self directed E learning module Theoretical knowledge and clinical competence Completion of questions Simulated practice assessment Register

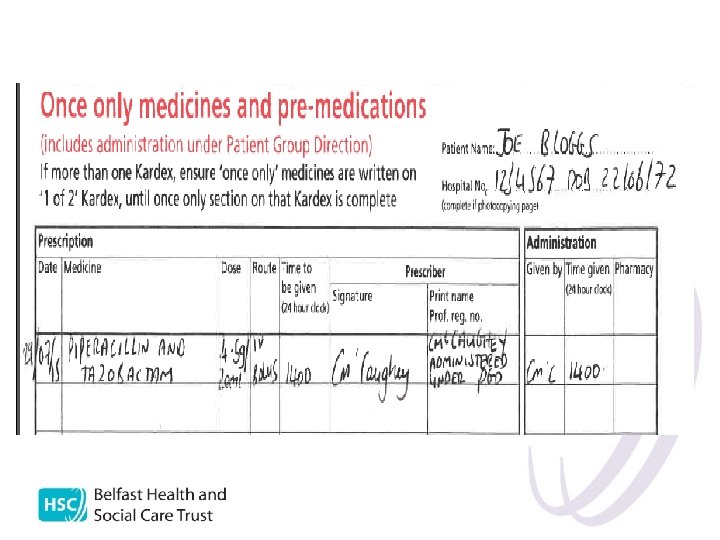
Record keeping l l l Record on patient kardex and on neutropenic sepsis pathway State: date, time, indication, drug, dose/volume, route, print name and sign State “administered under PGD” Document any adverse effects in notes A record must be kept of all patients treated under PGD for audit purposes
Pilot • Commenced 24 th August 2015 • 54% of staff trained and training ongoing • Since 2011 average 4 patients with NS a month • Therefore projected around 24 patients

• Eight patients in 6 week period • 1/3 of average NS episodes in last 4 years
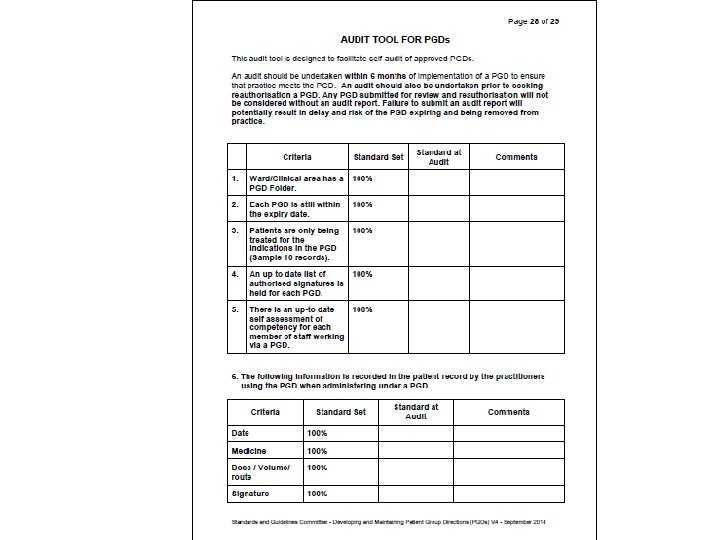

Results • Door to needle time audit - 100% compliance • PGD audit - 100% compliance
Conclusions and recommendations • Continue with PGD implementation • Extra focus on sepsis, secondary to PGD education, has perhaps aided 100% door to needle compliance • Continued implementation of PGD has potential to maintain this high standard

• Ciprofloxacin PGD (for Penicillin sensitive patients) written and ready for launch once Piperacillin and Tazobactam PGD evaluated • Also PGDs for saline flushes, Lidocaine, Chloramphenamine and Hydrocortisone (in event of hypersensitivity reaction) in final stages of development

References • NICE (2013) Medicines Practice Guidelines 2 PGDs www. nice. org. uk • NICE (2014) Competency framework: For health professionals using Patient Group Directions www. nice. org. uk • NICE (2015) PGD overview pathway www. nice. org. uk • NHS PGD website: http: //www. medicinesresources. nhs. uk/en/Communities/ NHS/PGDs/

caroline. mccaughey@qub. ac. uk
- Slides: 24