INTRODUCTION Many Are A ways to classify compounds















![� Square brackets are used to signify concentration, [H+], [OH–] � High [H+] = � Square brackets are used to signify concentration, [H+], [OH–] � High [H+] =](https://slidetodoc.com/presentation_image_h2/8851a4a9d43fd6958d442c4ce1cab9ef/image-27.jpg)

- Slides: 29


INTRODUCTION � Many � Are �A ways to classify compounds they ionic or covalent? common method is to separate into ACIDS and BASES

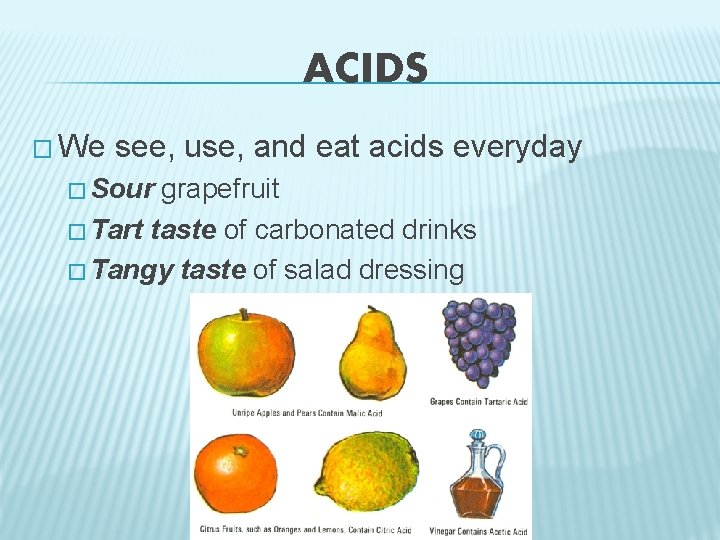
ACIDS � We see, use, and eat acids everyday � Sour grapefruit � Tart taste of carbonated drinks � Tangy taste of salad dressing

� Acidic juices are added to food to improve taste and help absorb nutrients � Stomach acid helps us digest food

ACIDS - 2 � Have corrosive properties � Acid rain can dissolve caves, buildings, and statues � Used to remove rust and purify/process metals

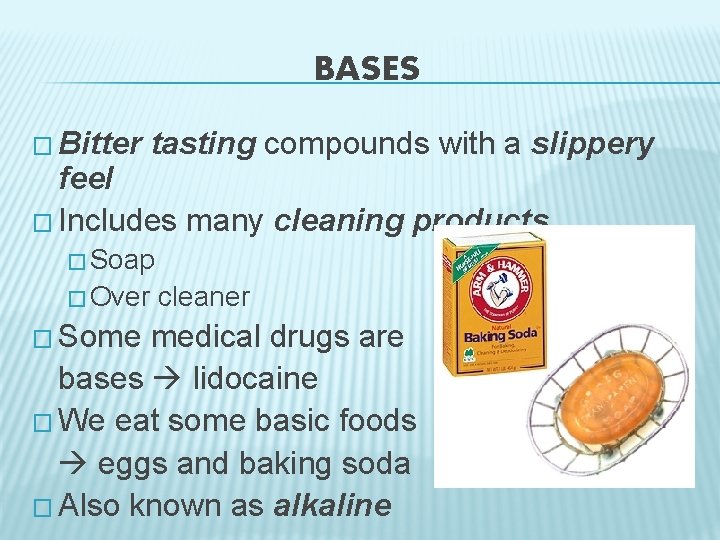
BASES � Bitter tasting compounds with a slippery feel � Includes many cleaning products � Soap � Over � Some cleaner medical drugs are bases lidocaine � We eat some basic foods eggs and baking soda � Also known as alkaline

SAFETY � Some acids and bases can be corrosive � Can burn throat or stomach if swallowed � Can burn skin or eyes on contact � NEVER attempt to identify an acid or base in the lab by taste or feel!

PH SCALE � p. H Scale: a number scale for measuring how acidic or how basic a solution is � Acids: have a p. H of less than 7 when dissolved in water � Bases: have a p. H of more than 7 when dissolved in water � Neutral: has a p. H of 7 (not an acid or a base)


PH OF COMMON SUBSTANCES � The more acidic a solution is, the lower the p. H � Lemon juice – p. H 2 � Tomato juice – p. H 4 � The more basic (or alkaline) a solution is, the higher the p. H � Think of alkaline Earth metals includes Ca and Mg which are basic when they react in water � Soap– p. H 10 � Oven Cleaner – p. H 13

� Neutral substances are neither acidic nor basic � Pure Water – p. H 7 � Saliva – p. H 6. 5 -7. 4 � Human blood – p. H of 7. 3 to 7. 5 (slightly basic)

USING THE PH SCALE � 1 unit of change represent a 10 times change in the degree of acidity/basicity Q: What is the increase in acidity if the p. H drops from 6 to 4? A: A 2 unit drop is a 10² or 100 times increase in acidity! IMPACT: Even a small increase in acidity harms coral reefs and organisms that require a specific p. H level to survive (ex. organisms that use calcium to make their shells)

PH INDICATORS � Many common acids and bases form colourless solutions (look like water) � p. H Indicators: chemicals that change colour depending on the p. H of the solution they’re placed in So what are some common indicators you could use?

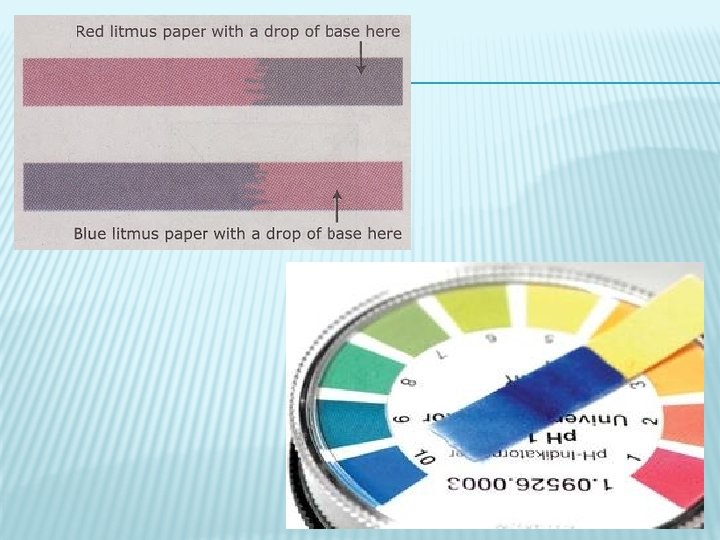
1. LITMUS PAPER � Litmus is extracted from lichens and dried onto thin paper strips � 2 forms: RED and BLUE � When BLUE litmus paper is placed in an acidic solution the paper will turn red � When RED litmus paper is placed in a basic solution it will turn blue � Can use both to tell if something is neutral BLUE will stay blue and RED will stay red HELPFUL HINT! � BAR = Blue + Acid Red


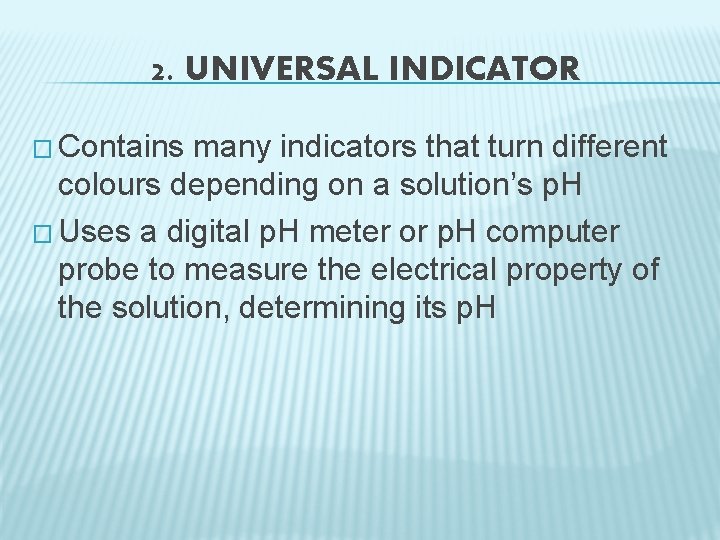
2. UNIVERSAL INDICATOR � Contains many indicators that turn different colours depending on a solution’s p. H � Uses a digital p. H meter or p. H computer probe to measure the electrical property of the solution, determining its p. H

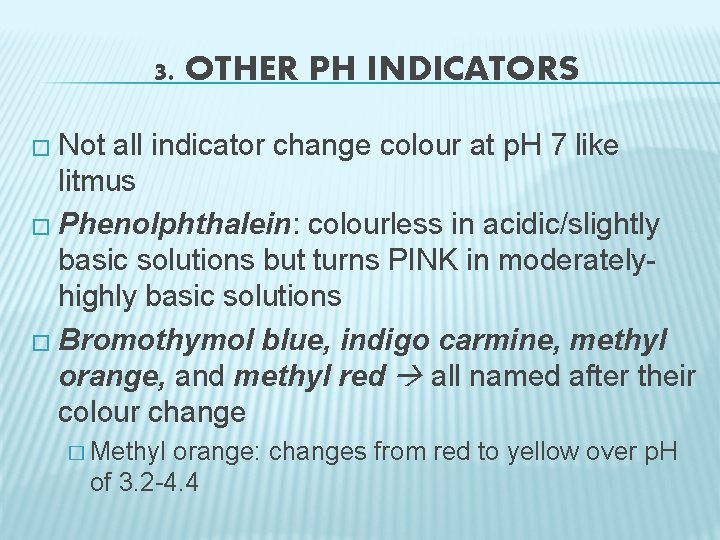
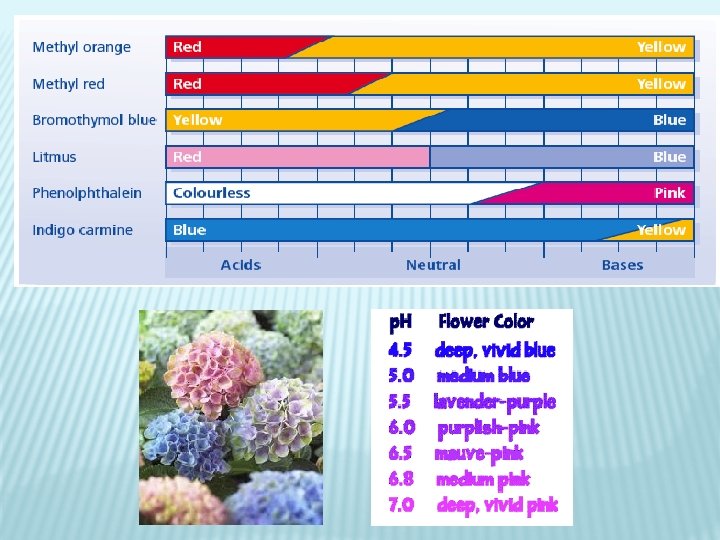
3. OTHER PH INDICATORS � Not all indicator change colour at p. H 7 like litmus � Phenolphthalein: colourless in acidic/slightly basic solutions but turns PINK in moderatelyhighly basic solutions � Bromothymol blue, indigo carmine, methyl orange, and methyl red all named after their colour change � Methyl orange: changes from red to yellow over p. H of 3. 2 -4. 4


ACIDS � Can sometimes identify acids by their chemical formulas � Many compounds (HCl) take on acid properties after mixing with water Ex. : HCl dissolved in water is written as HCl (aq), where (aq) means “aqueous” or “dissolved in water to make a solution

ACIDS - 2 � Chemical formulas usually have an “H” (hydrogen) on the left side of the formula HCl (aq) � In acids that contain CARBON, “H” may be written on the right side CH₃COOH (aq) � If no state of matter is given, the name may be given beginning with “Hydrogen” � Hydrogen chloride � If the acid is aqueous (ex. HCl (aq)), a different name is used that ends in “-ic acid” � Hydrochloric acid

NAMING ACIDS � Basic � pattern when “O” (oxygen) is present Names that begin in “hydrogen” and end in “-ate” are changed by dropping “hydrogen” from the name and changing the suffix to “-ic acid” Ex. : Hydrogen carbonate carbonic acid � Names that begin with “hydrogen” and end with “-ite” change by dropping the hydrogen and replacing the suffix with “-ous acid” Ex. : Hydrogen sulphite sulphurous acid

� Basic pattern when “O” (oxygen) is absent � Combine the 2 element names, then replace the ending with “-ic acid” � Ex. : hydrogen fluoride hydrofluoric acid � Ex. : hydrogen iodide hydriodic acid

The chemical formula of an acid usually starts with hydrogen (H). � Acids with a carbon usually have the C written first. �HCl(aq) = hydrochloric acid, HNO 3(aq) = nitric acid, CH 3 COOH(aq) = acetic acid � Naming acids � Hydrogen + …-ide = hydro…ic acid �HF(aq) = hydrogen fluoride = hydrofluoric acid � Hydrogen + …-ate = …ic acid �H 2 CO 3(aq) = hydrogen carbonate = carbonic acid � Hydrogen + …-ite = …ous acid �H SO = hydrogen sulphite = sulphurous �

BASES � Usually written with an “OH” on the right side of the formula � Some bases are much stronger than others � Magnesium hydroxide founds in antacids while sodium hydroxide is found in drain cleaner � Caustic: bases solutions made from highly reactive

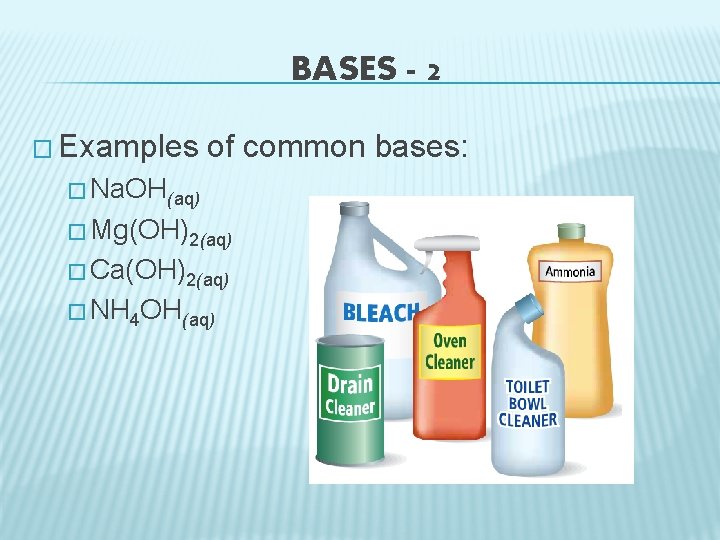
BASES - 2 � Examples of common bases: � Na. OH(aq) � Mg(OH)2(aq) � Ca(OH)2(aq) � NH 4 OH(aq)

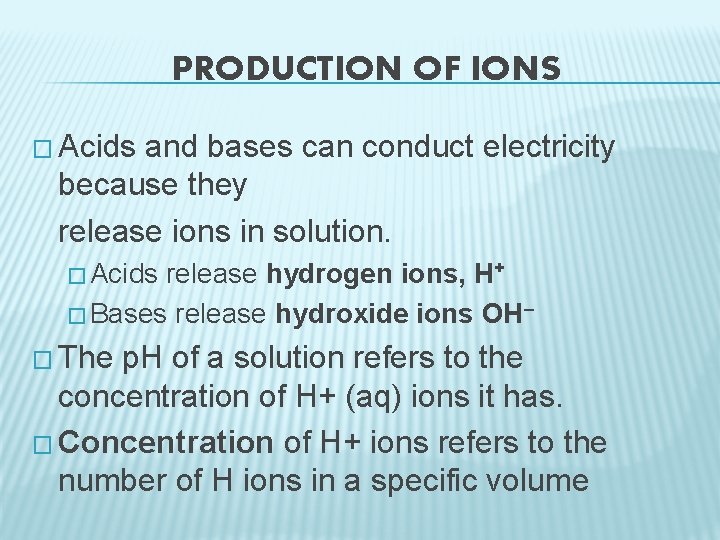
PRODUCTION OF IONS � Acids and bases can conduct electricity because they release ions in solution. � Acids release hydrogen ions, H+ � Bases release hydroxide ions OH– � The p. H of a solution refers to the concentration of H+ (aq) ions it has. � Concentration of H+ ions refers to the number of H ions in a specific volume
![Square brackets are used to signify concentration H OH High H � Square brackets are used to signify concentration, [H+], [OH–] � High [H+] =](https://slidetodoc.com/presentation_image_h2/8851a4a9d43fd6958d442c4ce1cab9ef/image-27.jpg)
� Square brackets are used to signify concentration, [H+], [OH–] � High [H+] = low p. H, very acidic � High [OH–] = high p. H, very basic �A solution cannot have BOTH high [H+] and [OH– ]; they cancel each other out and form water. This process is called neutralization. � H+ + OH– H 2 O

� Environmental � After Example: the mining of minerals, the remaining ground rock (tailings) are usually deposited in a tailings pond � The tailings can release acids, lowering the p. H of the water and affecting the environment � Can counteract this problem by adding a base to raise the p. H level back to normal

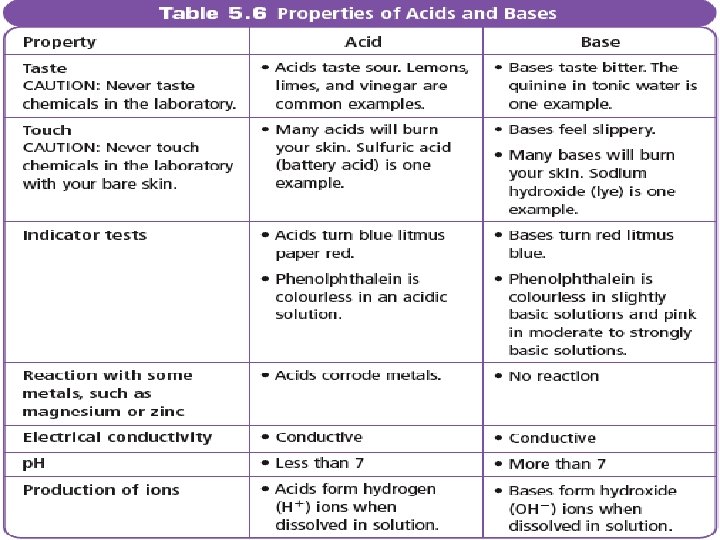
PROPERTIES OF ACIDS AND BASES