Introduction Into Biochemistry 1 What is Biochemistry Biochemistry
Introduction Into Biochemistry 1
What is Biochemistry? • Biochemistry = chemistry of life. • Biochemists use physical and chemical principles to explain biology at the molecular level. • Basic principles of biochemistry are common to all living organism 2
How does biochemistry impact you? • Medicine • Agriculture • Industrial applications • Environmental applications 3
Principle Areas of Biochemistry • Structure and function of biological macromolecules • Metabolism – anabolic and catabolic processes. • Molecular Genetics – How life is replicated. Regulation of protein synthesis 4

Origins of Biochemistry: A challenge to “Vitalism. ” Famous Dead Biochemist! 5
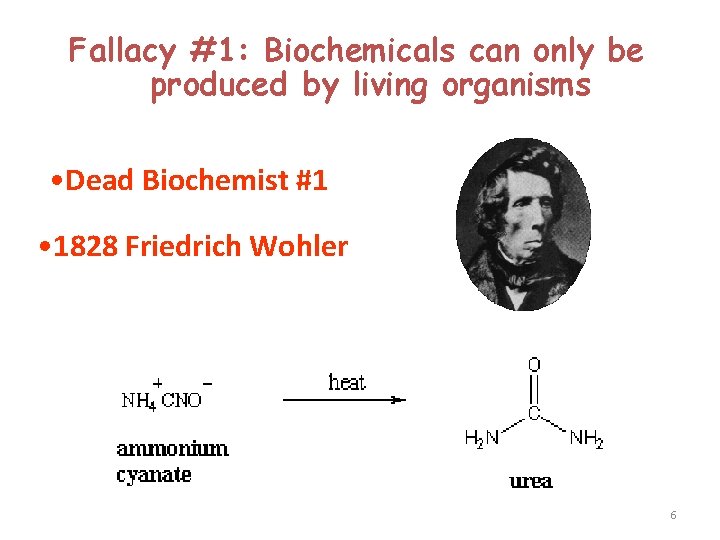
Fallacy #1: Biochemicals can only be produced by living organisms • Dead Biochemist #1 • 1828 Friedrich Wohler 6

Fallacy #2: Complex bioconversion of chemical substances require living matter Dead Biochemists #2 • 1897 Eduard Buchner Glucose + Dead Yeast = Alcohol 7
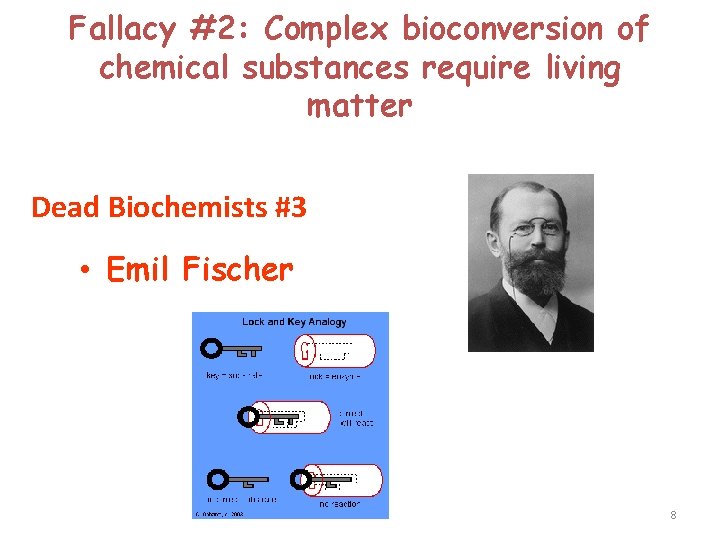
Fallacy #2: Complex bioconversion of chemical substances require living matter Dead Biochemists #3 • Emil Fischer 8
Organization of Life • elements • simple organic compounds (monomers) • macromolecules (polymers) • supramolecular structures • organelles • cells • tissues • organisms 9
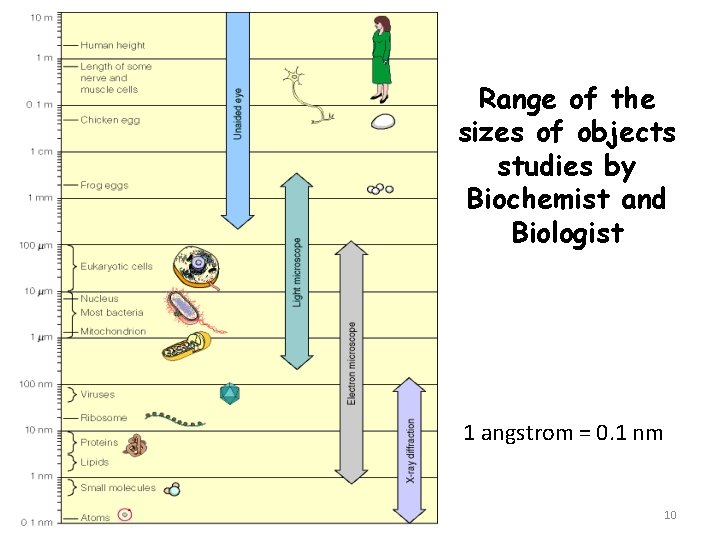
Range of the sizes of objects studies by Biochemist and Biologist 1 angstrom = 0. 1 nm 10
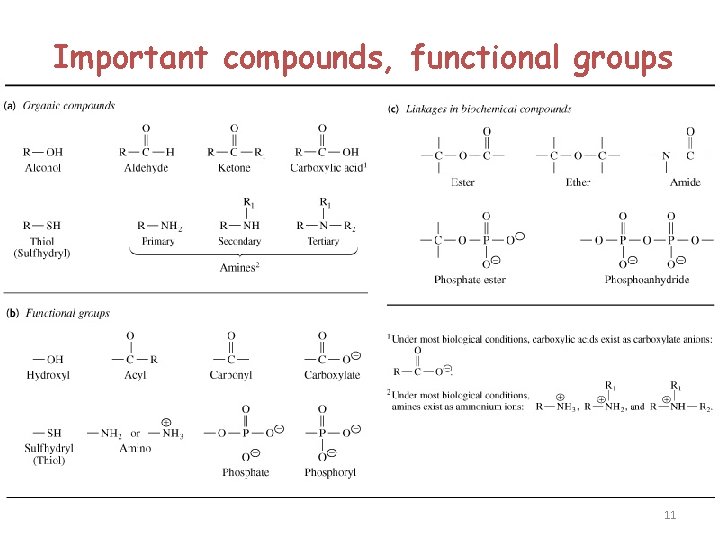
Important compounds, functional groups 11
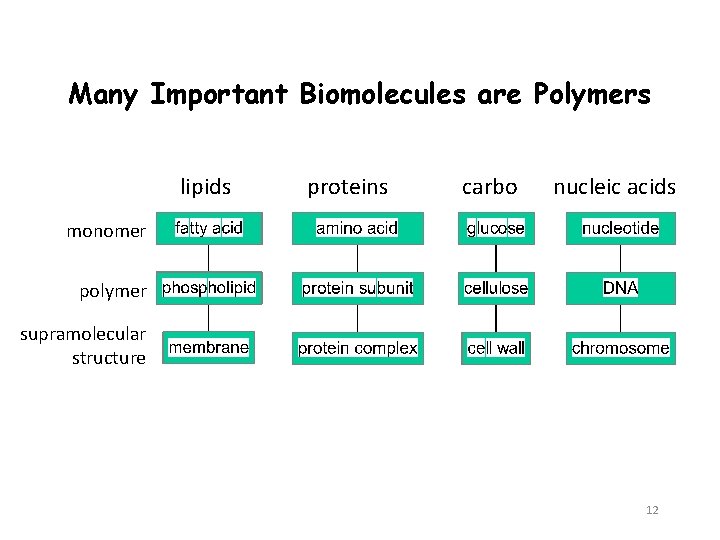
Many Important Biomolecules are Polymers lipids proteins carbo nucleic acids monomer polymer supramolecular structure 12
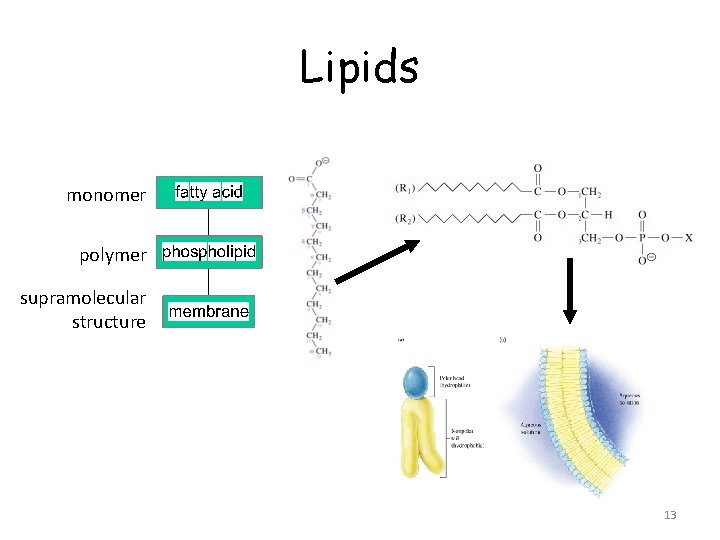
Lipids monomer polymer supramolecular structure 13
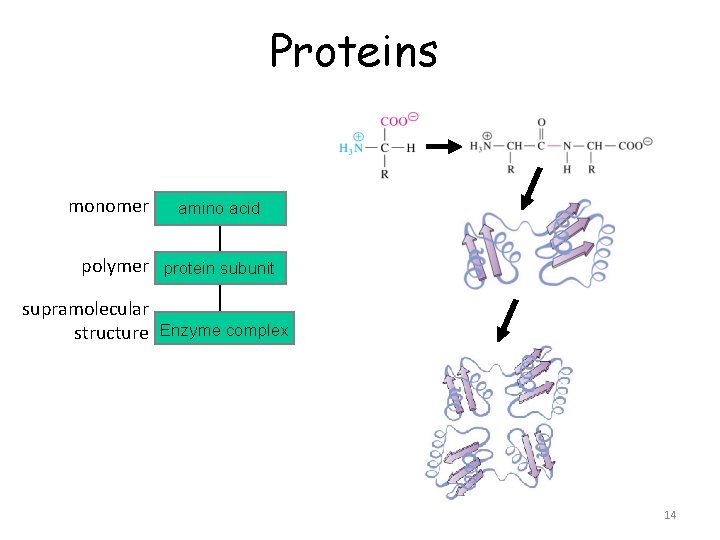
Proteins monomer amino acid polymer protein subunit supramolecular structure Enzyme complex 14
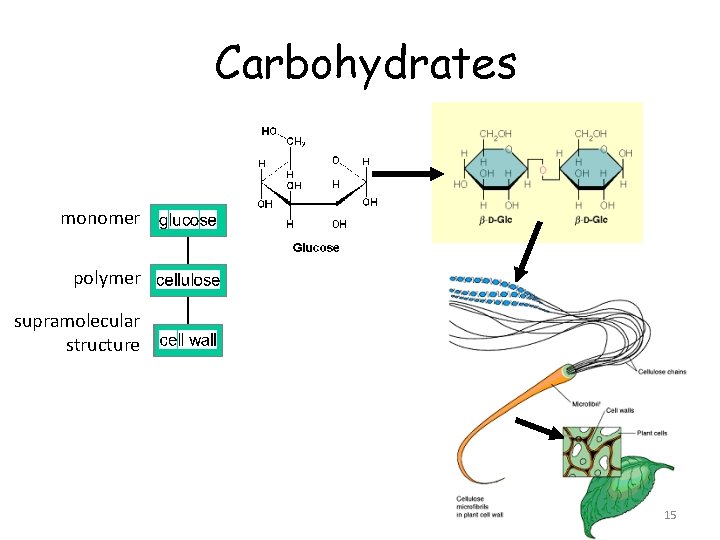
Carbohydrates monomer polymer supramolecular structure 15
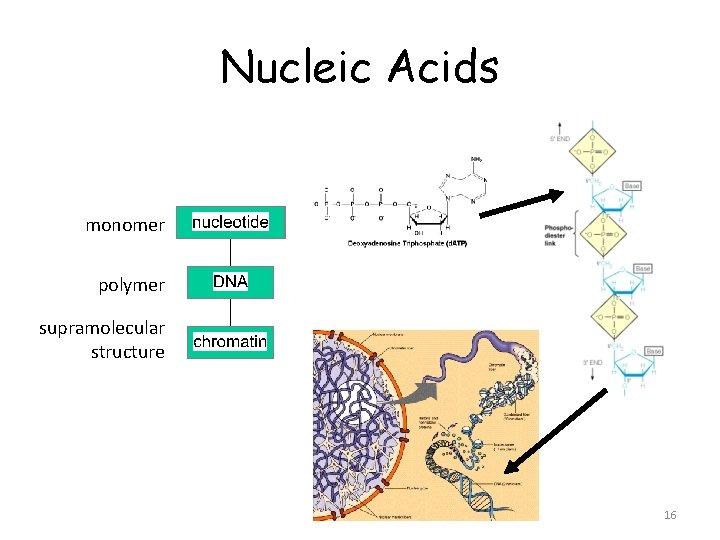
Nucleic Acids monomer polymer supramolecular structure 16
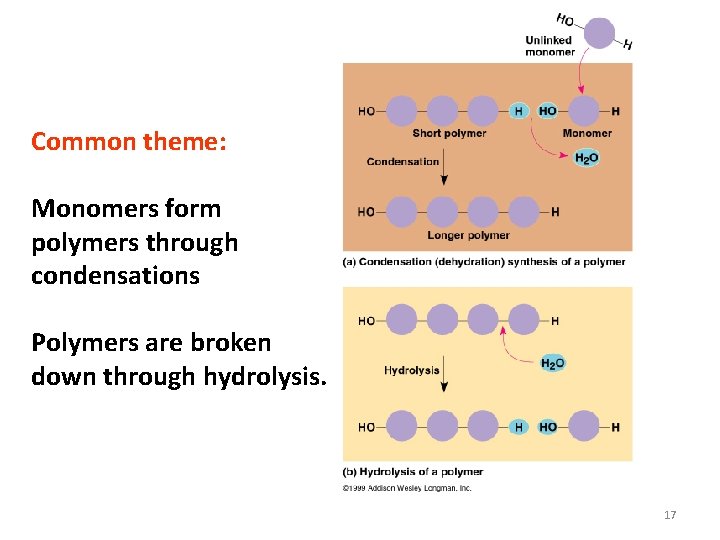
Common theme: Monomers form polymers through condensations Polymers are broken down through hydrolysis. 17
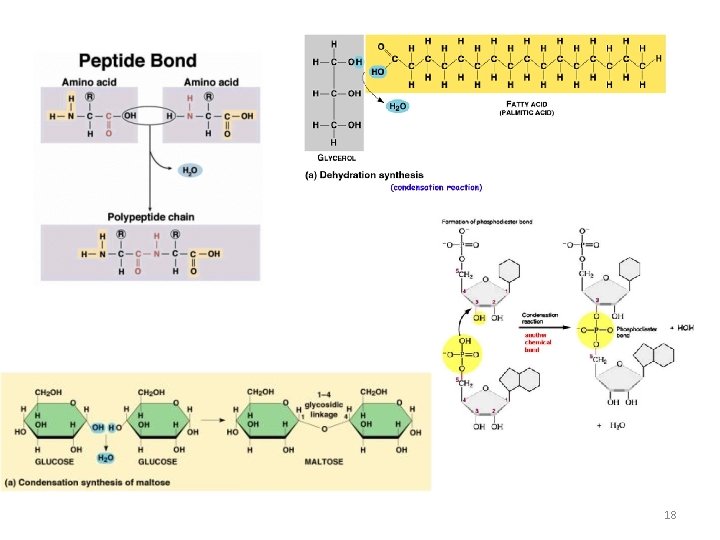
18
- Slides: 18