Introduction HPLC Process Lecture 1 Yuri Kazakevich Seton

- Slides: 23

Introduction HPLC Process Lecture 1 Yuri Kazakevich Seton Hall University 1

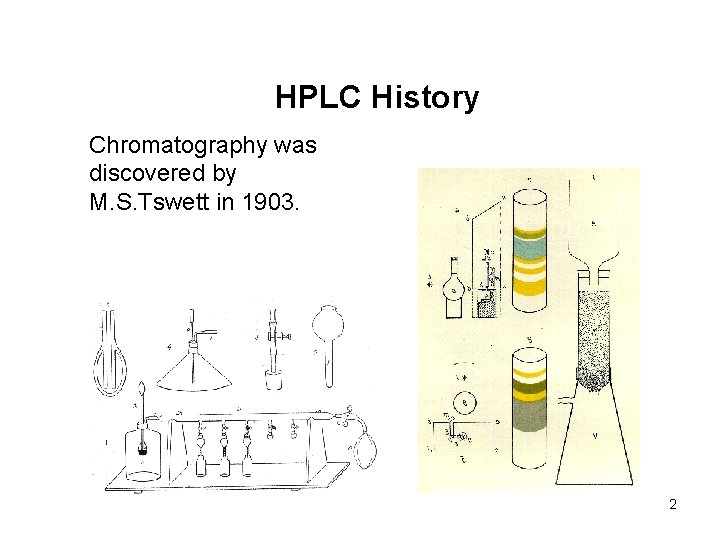
HPLC History Chromatography was discovered by M. S. Tswett in 1903. 2

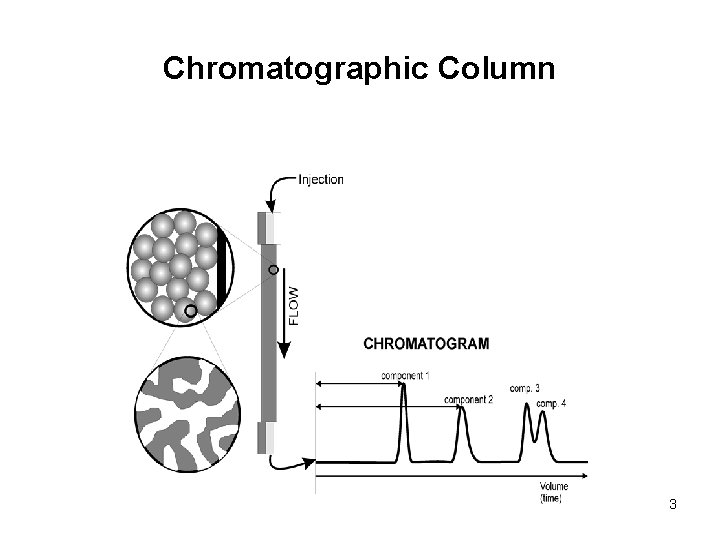
Chromatographic Column 3

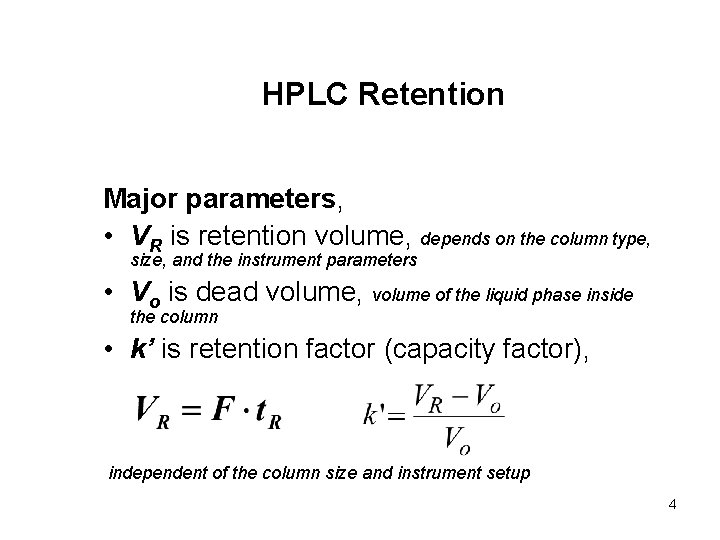
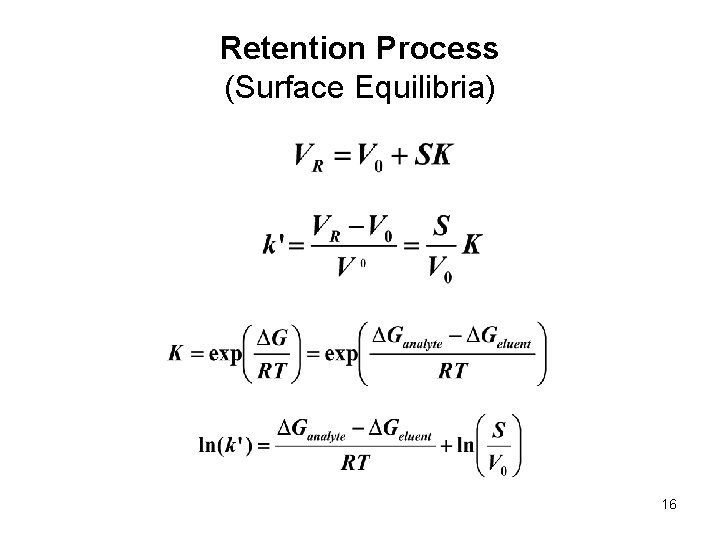
HPLC Retention Major parameters, • VR is retention volume, depends on the column type, size, and the instrument parameters • Vo is dead volume, volume of the liquid phase inside the column • k’ is retention factor (capacity factor), independent of the column size and instrument setup 4

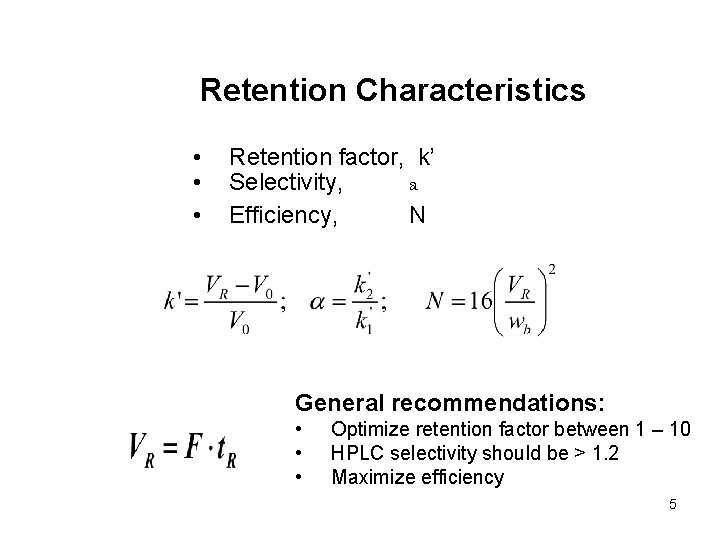
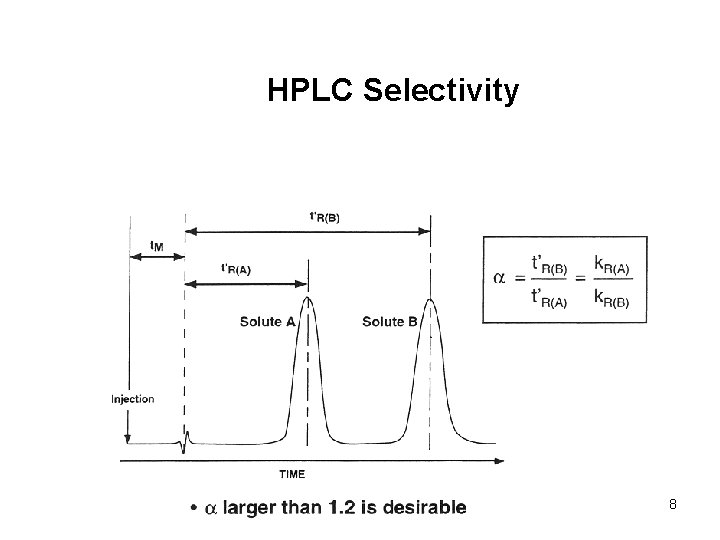
Retention Characteristics • • • Retention factor, k’ Selectivity, a Efficiency, N General recommendations: • • • Optimize retention factor between 1 – 10 HPLC selectivity should be > 1. 2 Maximize efficiency 5

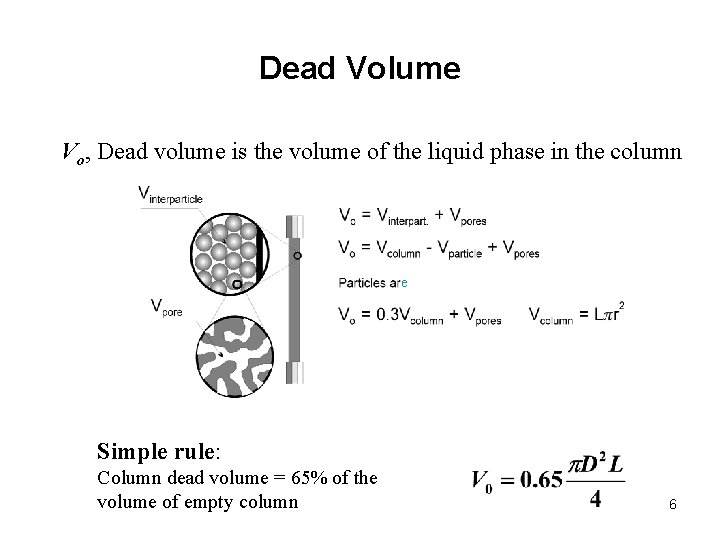
Dead Volume Vo, Dead volume is the volume of the liquid phase in the column e Simple rule: Column dead volume = 65% of the volume of empty column 6

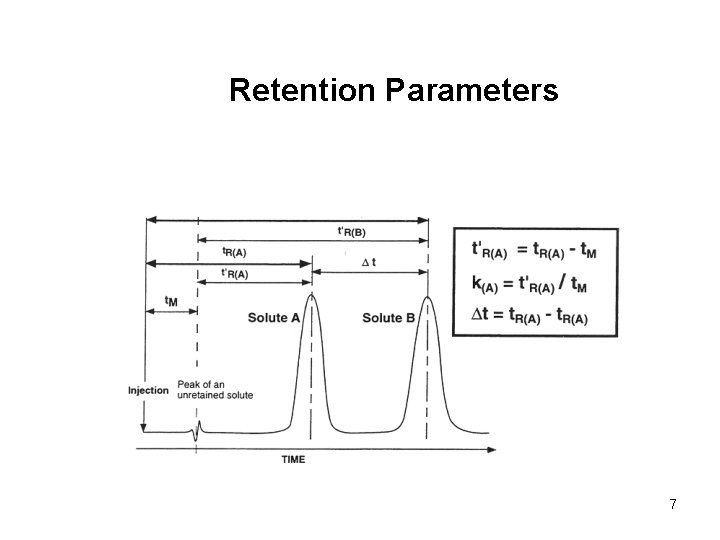
Retention Parameters 7

HPLC Selectivity 8

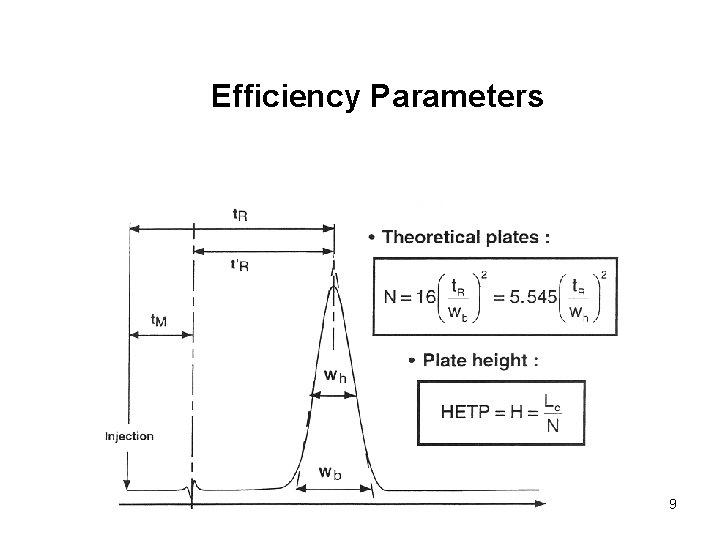
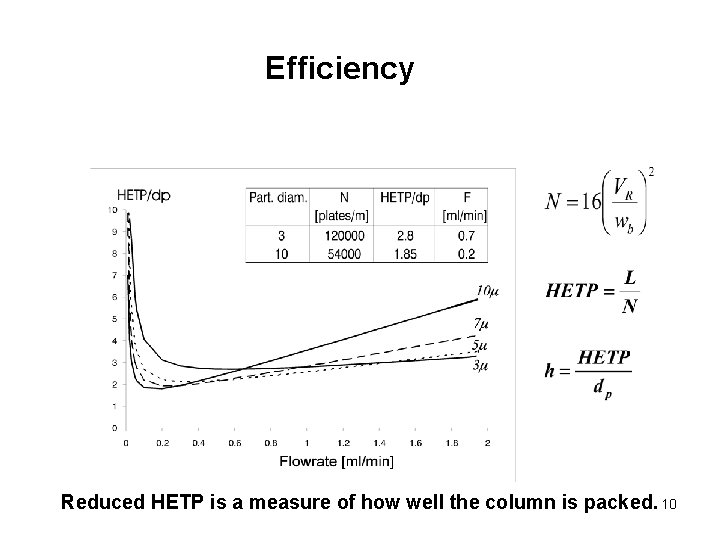
Efficiency Parameters 9

Efficiency Reduced HETP is a measure of how well the column is packed. 10

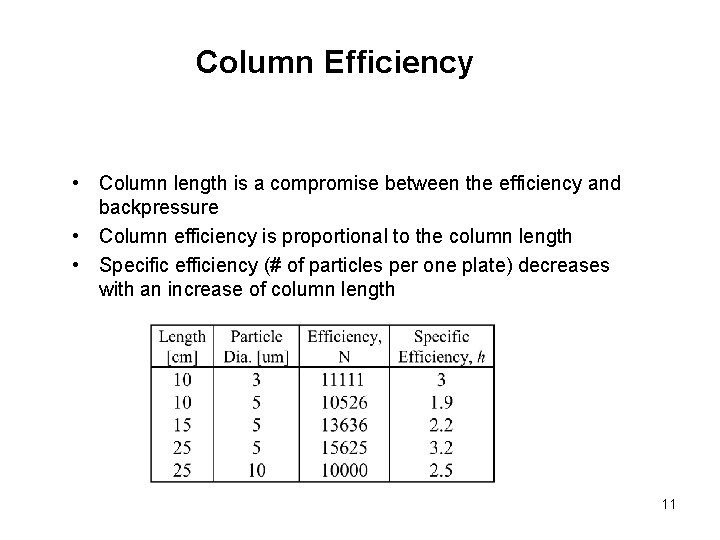
Column Efficiency • Column length is a compromise between the efficiency and backpressure • Column efficiency is proportional to the column length • Specific efficiency (# of particles per one plate) decreases with an increase of column length 11

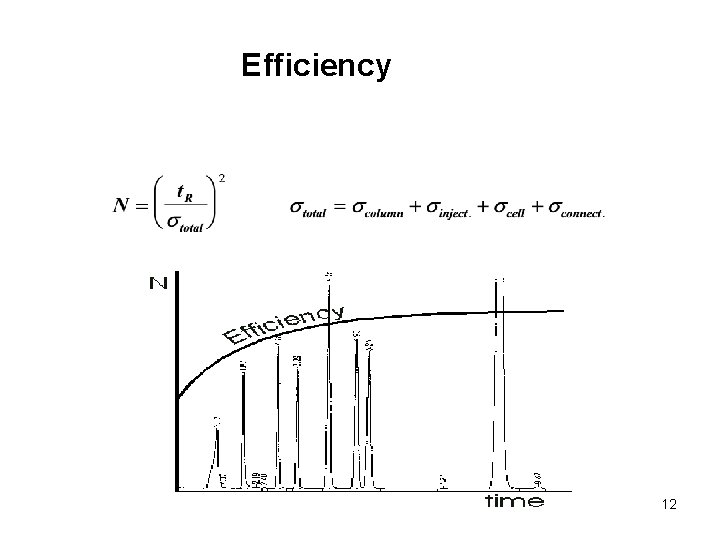
Efficiency 12

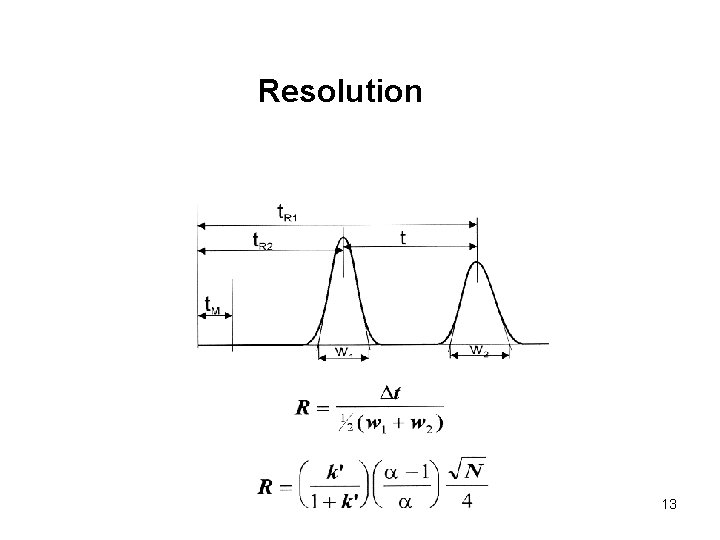
Resolution 13

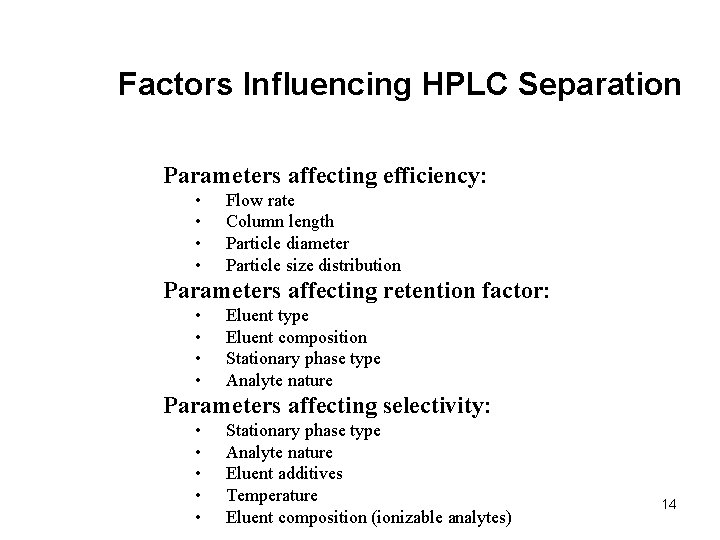
Factors Influencing HPLC Separation Parameters affecting efficiency: • • Flow rate Column length Particle diameter Particle size distribution Parameters affecting retention factor: • • Eluent type Eluent composition Stationary phase type Analyte nature Parameters affecting selectivity: • • • Stationary phase type Analyte nature Eluent additives Temperature Eluent composition (ionizable analytes) 14

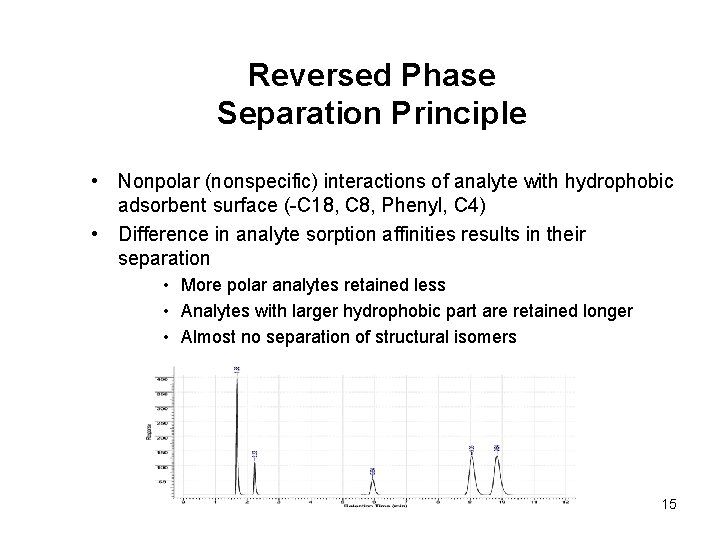
Reversed Phase Separation Principle • Nonpolar (nonspecific) interactions of analyte with hydrophobic adsorbent surface (-C 18, C 8, Phenyl, C 4) • Difference in analyte sorption affinities results in their separation • More polar analytes retained less • Analytes with larger hydrophobic part are retained longer • Almost no separation of structural isomers 15

Retention Process (Surface Equilibria) 16

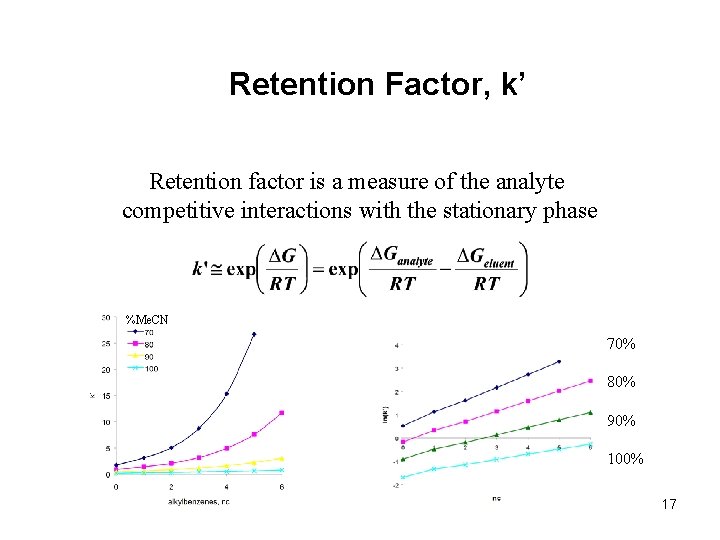
Retention Factor, k’ Retention factor is a measure of the analyte competitive interactions with the stationary phase %Me. CN 70% 80% 90% 100% 17

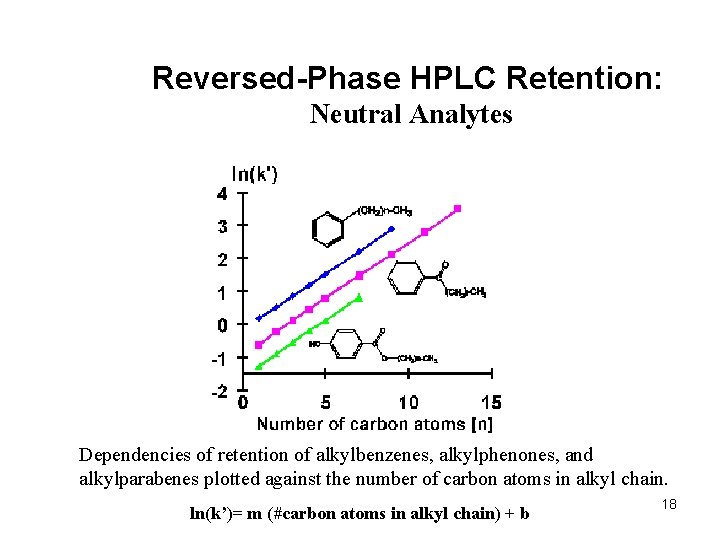
Reversed-Phase HPLC Retention: Neutral Analytes Dependencies of retention of alkylbenzenes, alkylphenones, and alkylparabenes plotted against the number of carbon atoms in alkyl chain. ln(k’)= m (#carbon atoms in alkyl chain) + b 18

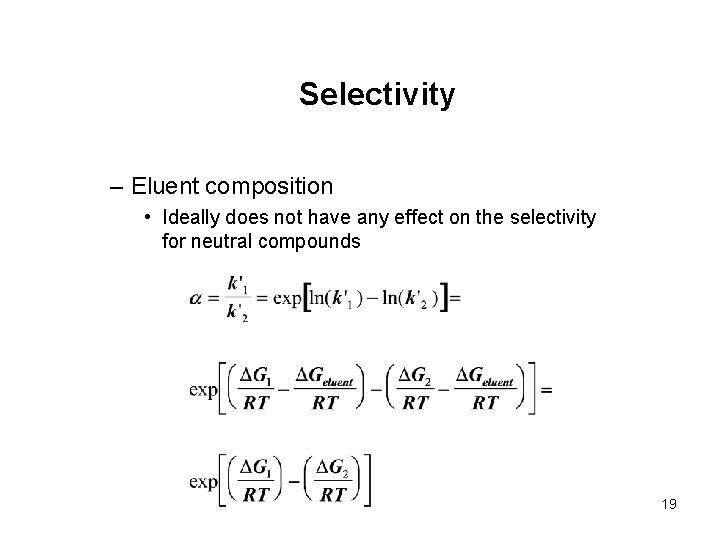
Selectivity – Eluent composition • Ideally does not have any effect on the selectivity for neutral compounds 19

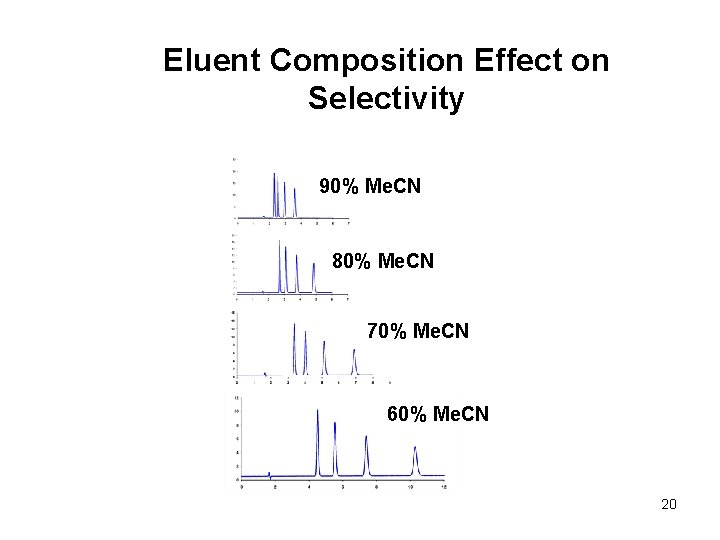
Eluent Composition Effect on Selectivity 90% Me. CN 80% Me. CN 70% Me. CN 60% Me. CN 20

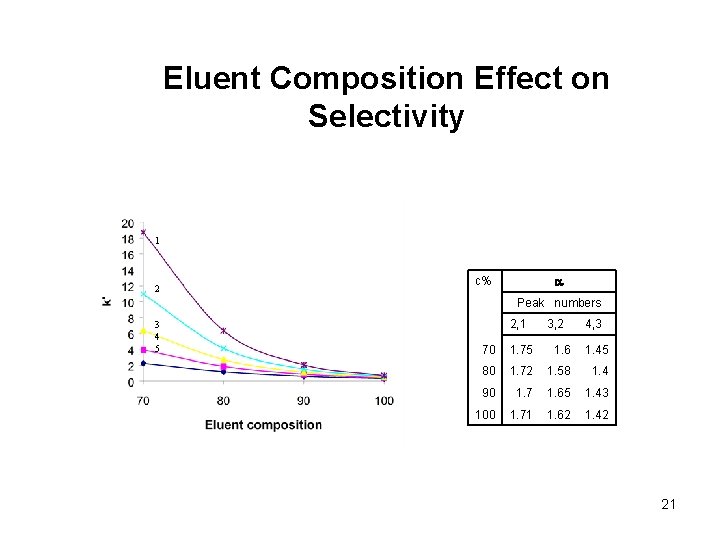
Eluent Composition Effect on Selectivity 1 2 a c% Peak numbers 3 4 5 2, 1 3, 2 4, 3 70 1. 75 1. 6 1. 45 80 1. 72 1. 58 1. 4 90 1. 7 1. 65 1. 43 100 1. 71 1. 62 1. 42 21

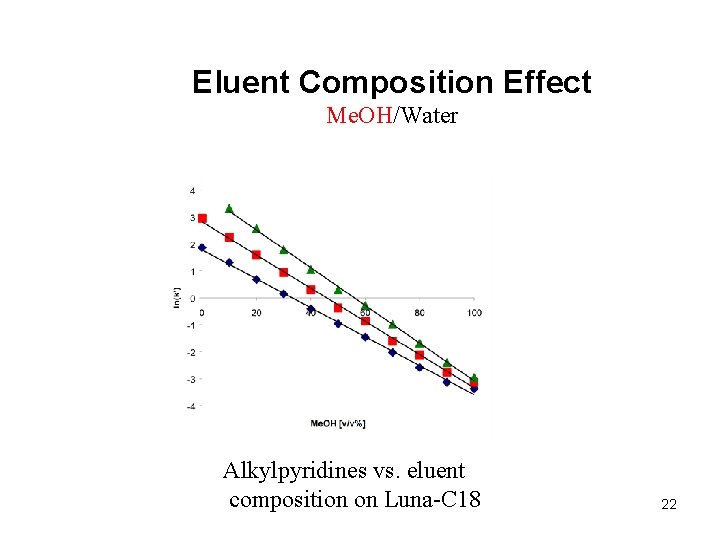
Eluent Composition Effect Me. OH/Water Alkylpyridines vs. eluent composition on Luna-C 18 22

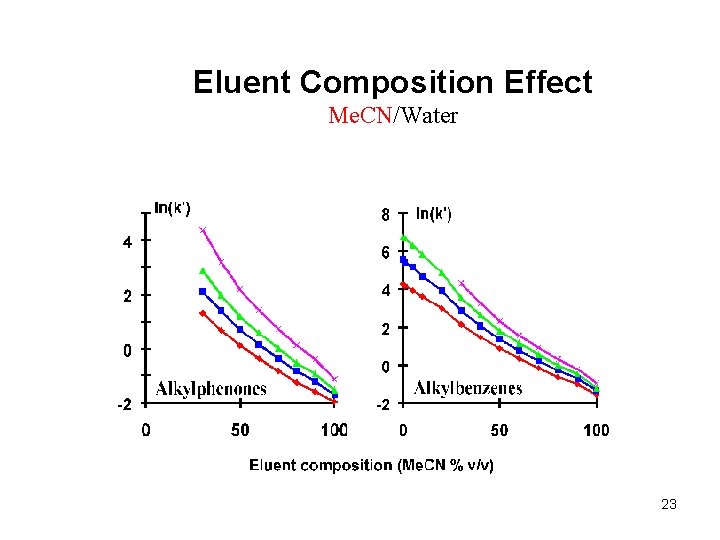
Eluent Composition Effect Me. CN/Water 23