Introducing stem cells Stem cell biology basics IVF
Introducing stem cells

Stem cell biology basics
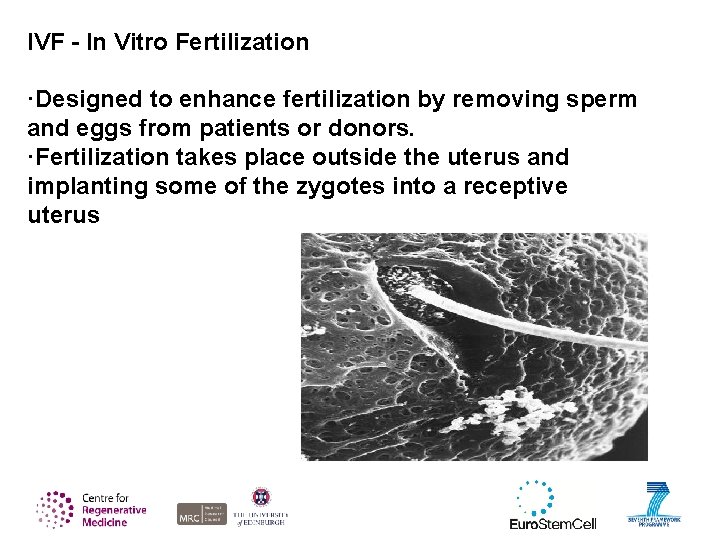
IVF - In Vitro Fertilization ·Designed to enhance fertilization by removing sperm and eggs from patients or donors. ·Fertilization takes place outside the uterus and implanting some of the zygotes into a receptive uterus

The world's first IVF baby Louise Brown (2 nd right) with her son Cameron, her mother Lesley and IVF pioneer Professor Robert Edwards. The procedure was done in London, 1978.

IVF is now a very common practice although not without risk ·mutation, miscarriage etc. Commercially it is used to produce cattle ·transporting frozen zygotes is easier and less expensive than transporting live cattle
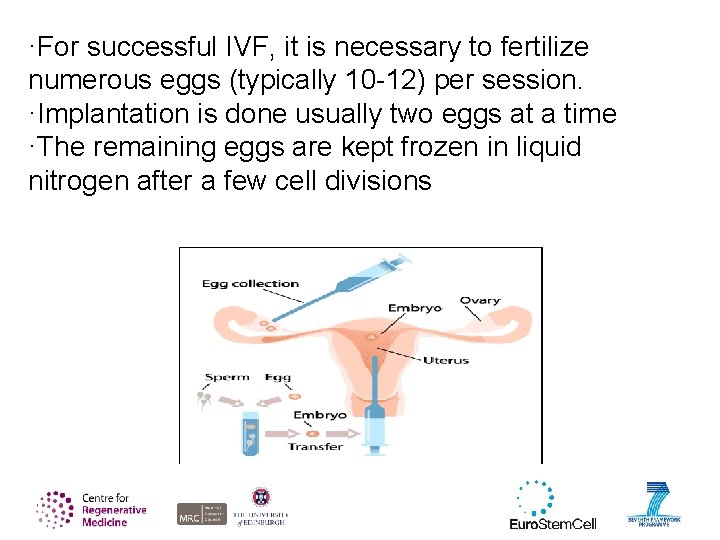
·For successful IVF, it is necessary to fertilize numerous eggs (typically 10 -12) per session. ·Implantation is done usually two eggs at a time ·The remaining eggs are kept frozen in liquid nitrogen after a few cell divisions

·While freezing zygotes is an acceptable practice, the effects on the embryo are still unknown ·The majority of frozen zygotes that are then implanted into healthy women develop into healthy children, however, a large number of zygotes do not survive the freezing process. - Parents can choose to have their eggs frozen indefinitely, incinerated, or donated to scientific research like human embryonic stem cell lines.
Of the estimated 400, 000 frozen embryos in the U. S. less than 3% have been donated to scientific research.
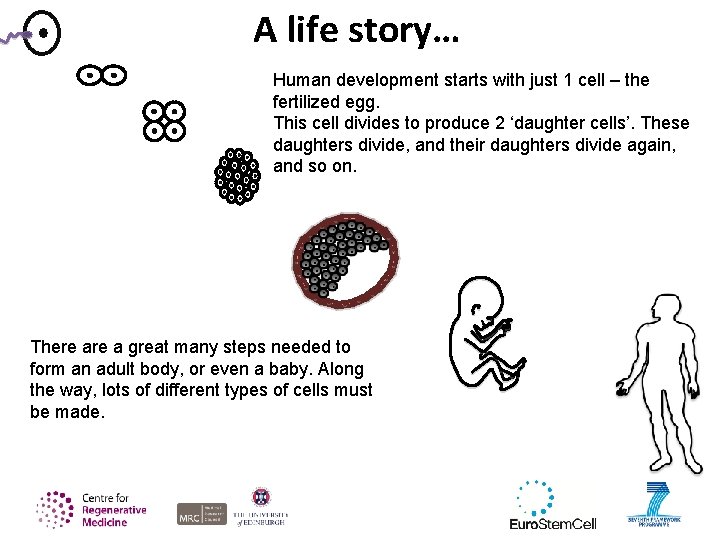
A life story… Human development starts with just 1 cell – the fertilized egg. This cell divides to produce 2 ‘daughter cells’. These daughters divide, and their daughters divide again, and so on. There a great many steps needed to form an adult body, or even a baby. Along the way, lots of different types of cells must be made.
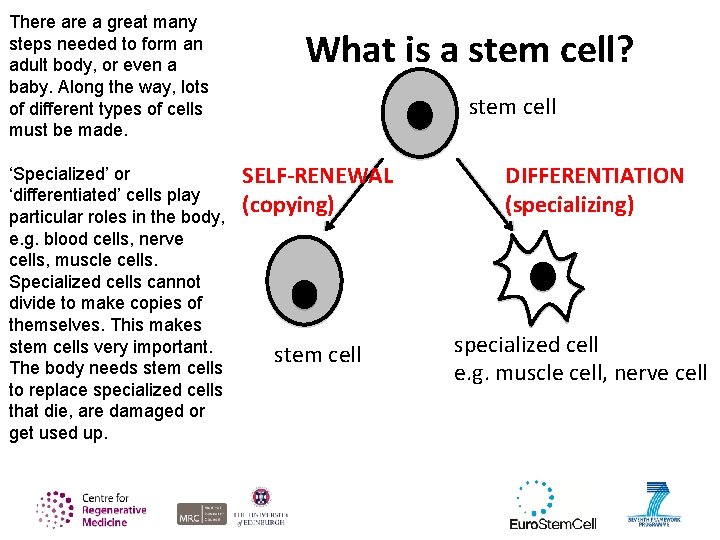
There a great many steps needed to form an adult body, or even a baby. Along the way, lots of different types of cells must be made. ‘Specialized’ or ‘differentiated’ cells play particular roles in the body, e. g. blood cells, nerve cells, muscle cells. Specialized cells cannot divide to make copies of themselves. This makes stem cells very important. The body needs stem cells to replace specialized cells that die, are damaged or get used up. What is a stem cell? stem cell SELF-RENEWAL (copying) stem cell DIFFERENTIATION (specializing) specialized cell e. g. muscle cell, nerve cell
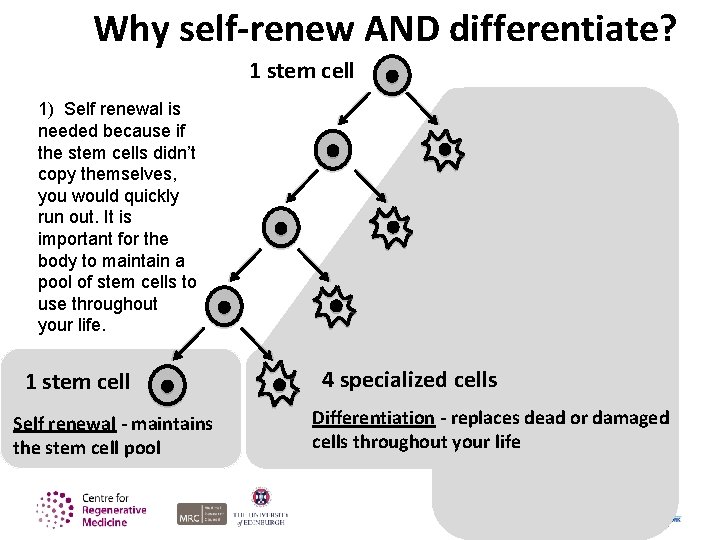
Why self-renew AND differentiate? 1 stem cell 1) Self renewal is needed because if the stem cells didn’t copy themselves, you would quickly run out. It is important for the body to maintain a pool of stem cells to use throughout your life. 1 stem cell Self renewal - maintains the stem cell pool 4 specialized cells Differentiation - replaces dead or damaged cells throughout your life
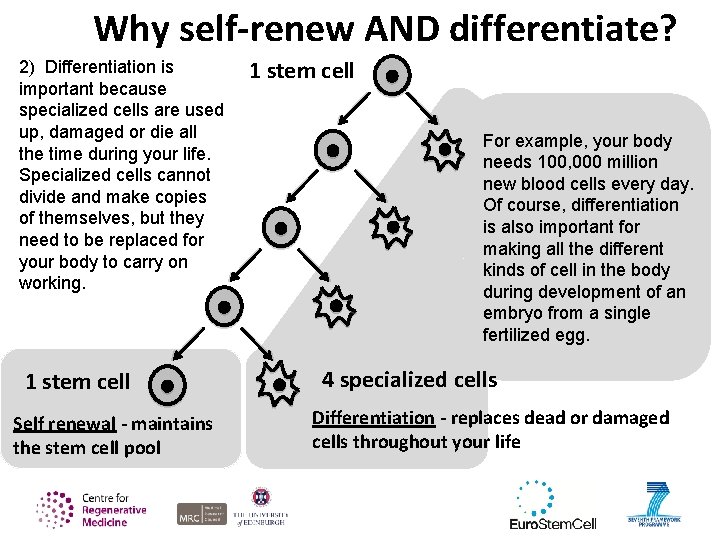
Why self-renew AND differentiate? 2) Differentiation is important because specialized cells are used up, damaged or die all the time during your life. Specialized cells cannot divide and make copies of themselves, but they need to be replaced for your body to carry on working. 1 stem cell Self renewal - maintains the stem cell pool 1 stem cell For example, your body needs 100, 000 million new blood cells every day. Of course, differentiation is also important for making all the different kinds of cell in the body during development of an embryo from a single fertilized egg. 4 specialized cells Differentiation - replaces dead or damaged cells throughout your life
Types of stem cell: 1) Embryonic stem cells
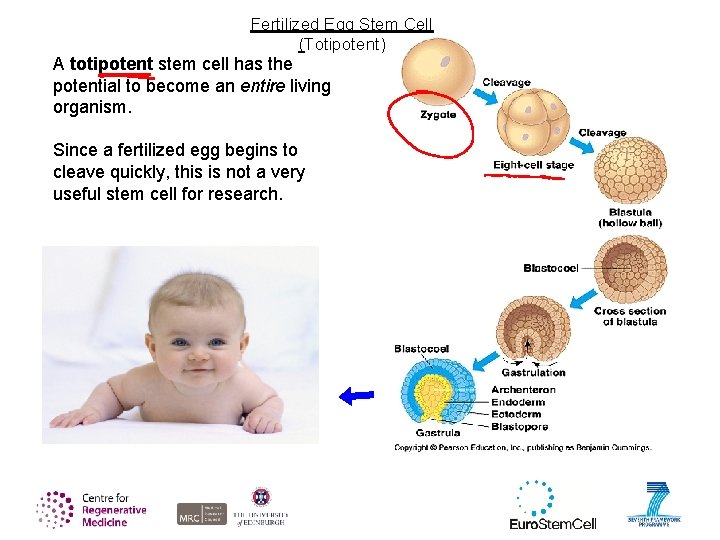
Fertilized Egg Stem Cell (Totipotent) A totipotent stem cell has the potential to become an entire living organism. Since a fertilized egg begins to cleave quickly, this is not a very useful stem cell for research.
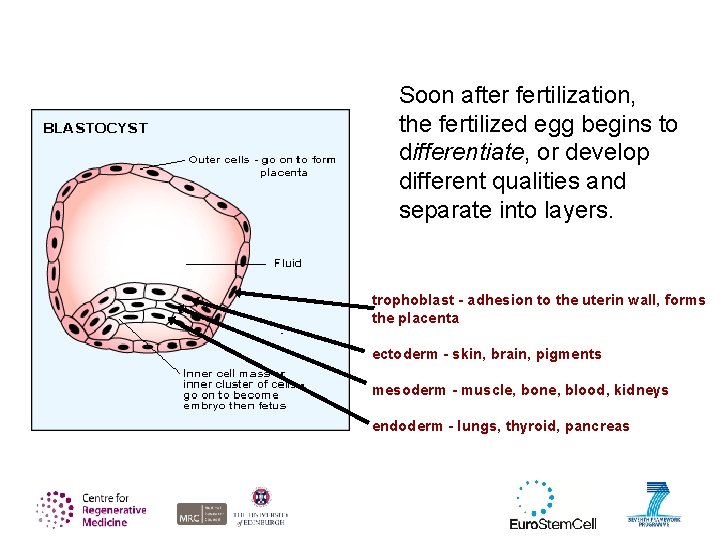
Soon after fertilization, the fertilized egg begins to differentiate, or develop different qualities and separate into layers. trophoblast - adhesion to the uterin wall, forms the placenta ectoderm - skin, brain, pigments mesoderm - muscle, bone, blood, kidneys endoderm - lungs, thyroid, pancreas
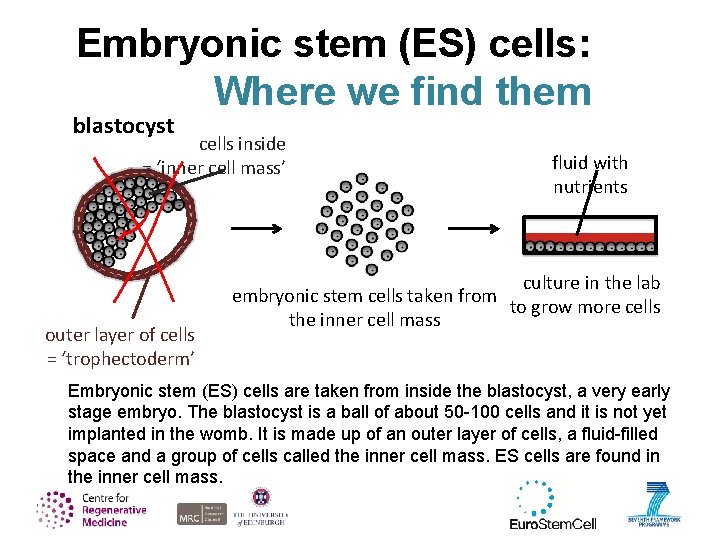
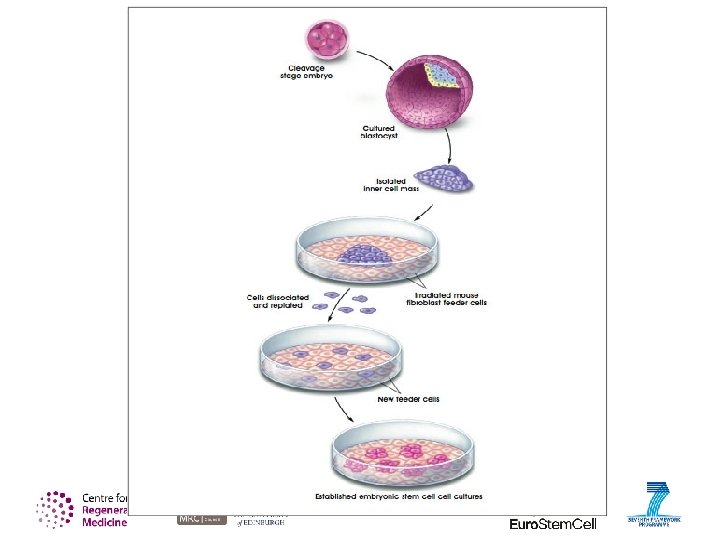
Embryonic stem (ES) cells: Where we find them blastocyst cells inside = ‘inner cell mass’ outer layer of cells = ‘trophectoderm’ fluid with nutrients culture in the lab embryonic stem cells taken from to grow more cells the inner cell mass Embryonic stem (ES) cells are taken from inside the blastocyst, a very early stage embryo. The blastocyst is a ball of about 50 -100 cells and it is not yet implanted in the womb. It is made up of an outer layer of cells, a fluid-filled space and a group of cells called the inner cell mass. ES cells are found in the inner cell mass.
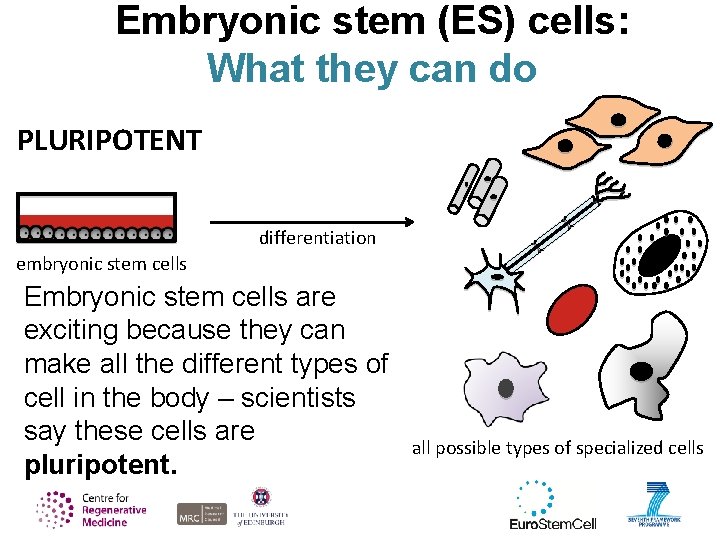
Embryonic stem (ES) cells: What they can do PLURIPOTENT differentiation embryonic stem cells Embryonic stem cells are exciting because they can make all the different types of cell in the body – scientists say these cells are pluripotent. all possible types of specialized cells
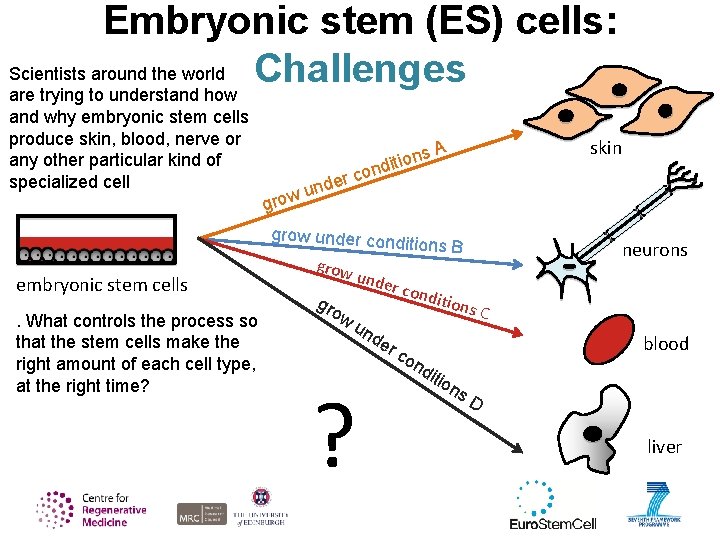
Embryonic stem (ES) cells: Scientists around the world Challenges are trying to understand how and why embryonic stem cells produce skin, blood, nerve or any other particular kind of specialized cell grow er und ns o i t i nd skin A co grow under cond embryonic stem cells. What controls the process so that the stem cells make the right amount of each cell type, at the right time? grow gro w itions B neurons unde r con un de r ? ditio ns C blood co nd itio ns D liver
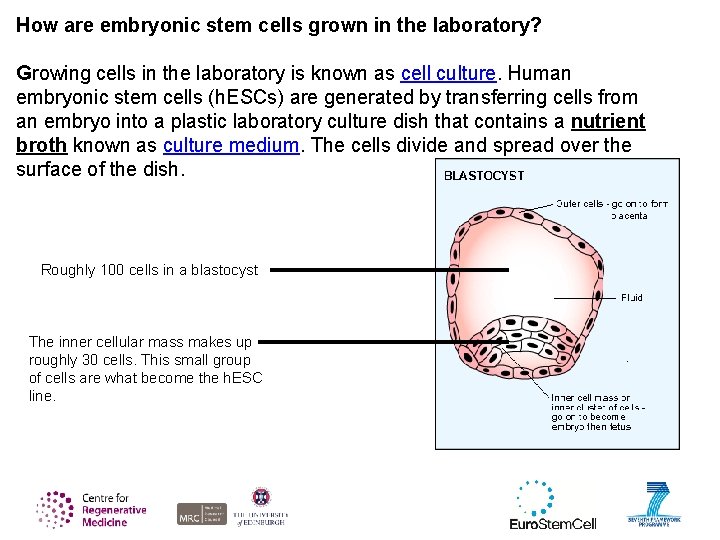
How are embryonic stem cells grown in the laboratory? Growing cells in the laboratory is known as cell culture. Human embryonic stem cells (h. ESCs) are generated by transferring cells from an embryo into a plastic laboratory culture dish that contains a nutrient broth known as culture medium. The cells divide and spread over the surface of the dish. Roughly 100 cells in a blastocyst The inner cellular mass makes up roughly 30 cells. This small group of cells are what become the h. ESC line.
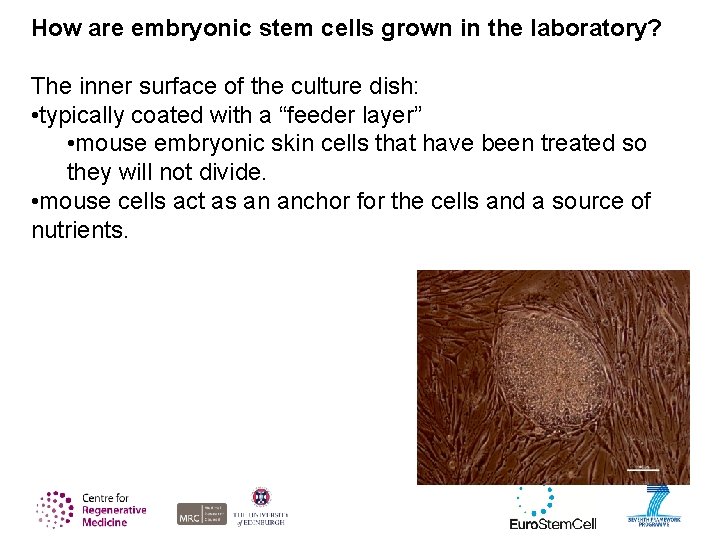
How are embryonic stem cells grown in the laboratory? The inner surface of the culture dish: • typically coated with a “feeder layer” • mouse embryonic skin cells that have been treated so they will not divide. • mouse cells act as an anchor for the cells and a source of nutrients.
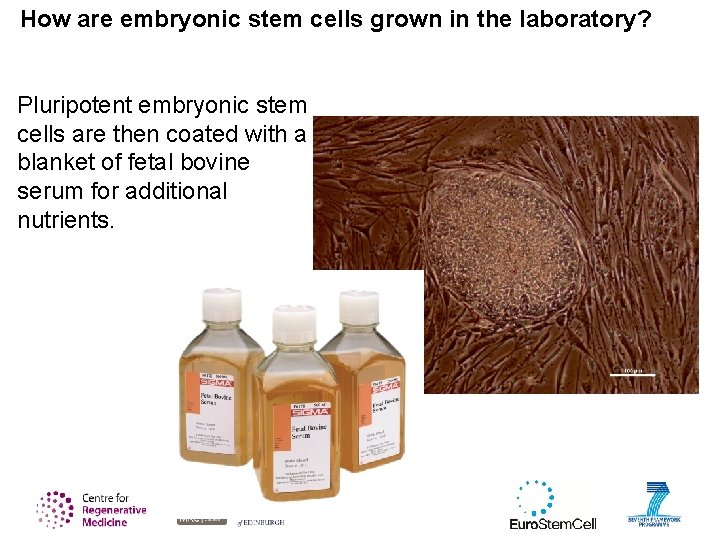
How are embryonic stem cells grown in the laboratory? Pluripotent embryonic stem cells are then coated with a blanket of fetal bovine serum for additional nutrients.
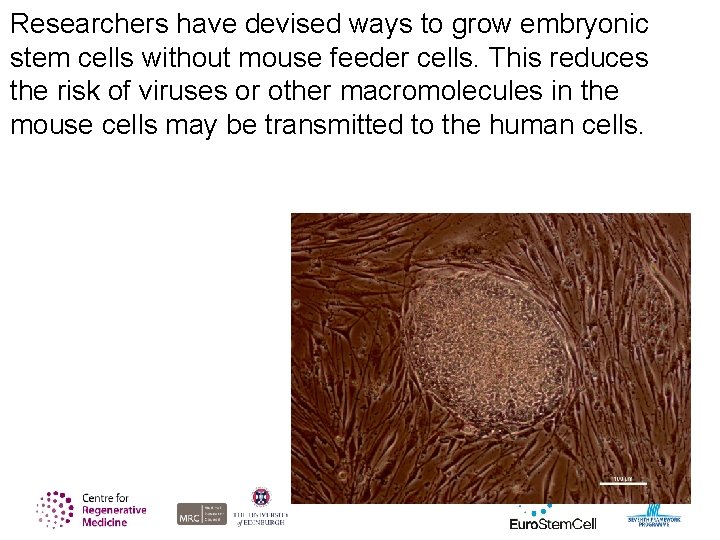
Researchers have devised ways to grow embryonic stem cells without mouse feeder cells. This reduces the risk of viruses or other macromolecules in the mouse cells may be transmitted to the human cells.
Stem Cell Line The process of generating an embryonic stem cell line is somewhat inefficient, often, the plated cells do not survive. However, if the plated cells survive, divide and multiply enough to crowd the dish, they are removed gently and plated into several fresh culture dishes. Before 2009, only 22 h. ESC lines had been established. After George W. Bush's ban on federally funded h. ESCs, only these 22 lines were legally allowed to be used for research.
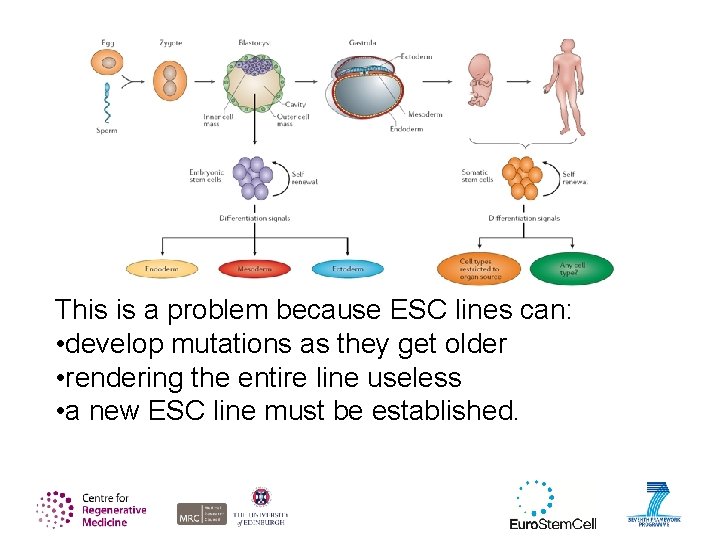
This is a problem because ESC lines can: • develop mutations as they get older • rendering the entire line useless • a new ESC line must be established.
Culturing embryonic stem cells has many difficulties. Teratomas - typically a tumor of randomly differentiated or partly differentiated cell types But teratomas have their uses. Teratomas are used as an indication that the embryonic stem cells are capable of differentiating into multiple cell types.
Embryonic stem (ES) cells: Challenges Learning to control these fascinating cells is a big challenge. If we could control their differentiation, we would have a powerful tool for developing treatments for disease. For example: • growing new insulin-producing cells to transplant into a patient with diabetes. • Understand how diseases develop (disease modeling) • Test drugs in the laboratory

Embryonic stem (ES) cells: Challenges - Ethics • human embryonic stem cells come from unused IVF eggs donated by the parents. • They are not harvested from aborted fetuses. • Does this constitute an ethical dilemma? • Are embryos considered human life or a collection of human cells? • Should research continue with h. ESCs?
Embryonic stem (ES) cells: Challenges - Funding Federal v. Privately Funded SC Research Private Pros ·Private companies tend to produce results faster ·Competition between rival companies can drive new discoveries and research methods/tools ·Funds for research can be procured from a wide variety of sources (investors, research grants venture capitalists etc. ) Private Cons ·Faster results can mean sloppy or less detailed results ·Competition means a lack of sharing information which can often slow down discoveries ·Profit-driven motives could lead to less attention being paid to safety and efficacy

Embryonic stem (ES) cells: Challenges - Funding Federal v. Privately Funded SC Research Federal Pros ·Federal researchers tend to produce more reliable results ·Federal researchers tend to share discoveries through published papers ·Having a single source of funds (taxpayer money) can help researchers focus on the science rather than the profit Federal Cons ·Research tends to take longer due to federal bureaucracy ·Researchers must deal with controversial subjects using taxpayer money leading to a stalling or complete block to reaserch due to a changing political climate.
Types of stem cell: 2) Tissue stem cells
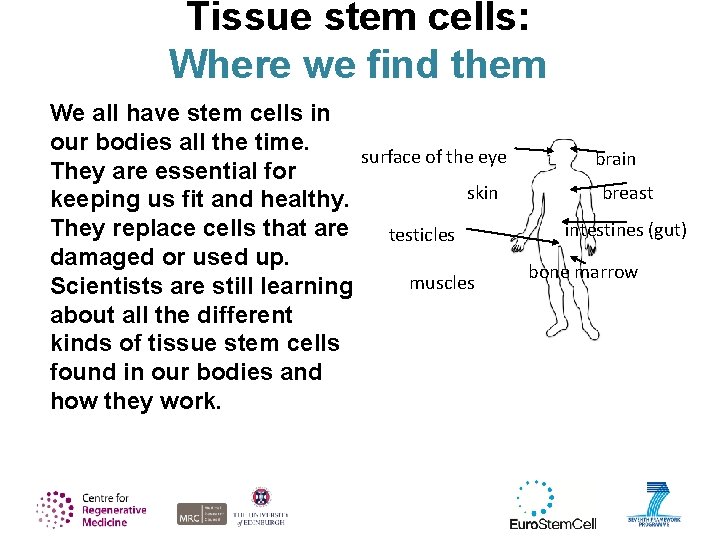
Tissue stem cells: Where we find them We all have stem cells in our bodies all the time. surface of the eye brain They are essential for breast skin keeping us fit and healthy. They replace cells that are intestines (gut) testicles damaged or used up. bone marrow muscles Scientists are still learning about all the different kinds of tissue stem cells found in our bodies and how they work.
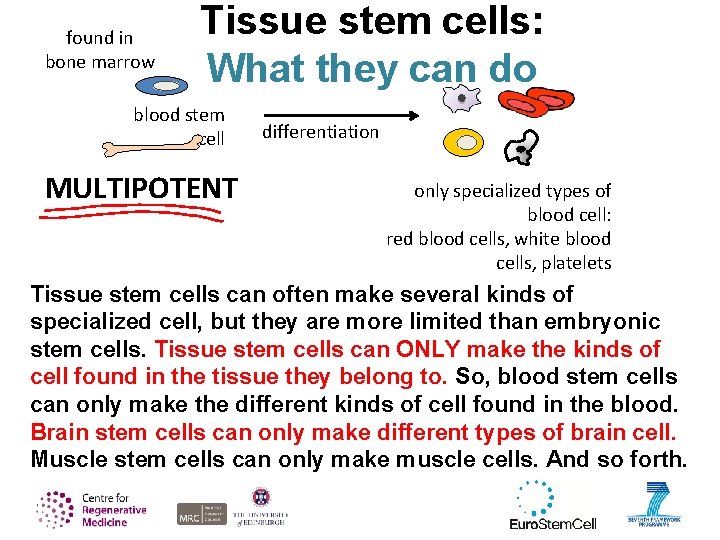
found in bone marrow Tissue stem cells: What they can do blood stem cell MULTIPOTENT differentiation only specialized types of blood cell: red blood cells, white blood cells, platelets Tissue stem cells can often make several kinds of specialized cell, but they are more limited than embryonic stem cells. Tissue stem cells can ONLY make the kinds of cell found in the tissue they belong to. So, blood stem cells can only make the different kinds of cell found in the blood. Brain stem cells can only make different types of brain cell. Muscle stem cells can only make muscle cells. And so forth.
Types of stem cell: 3)Induced pluripotent (i. PS) stem cells
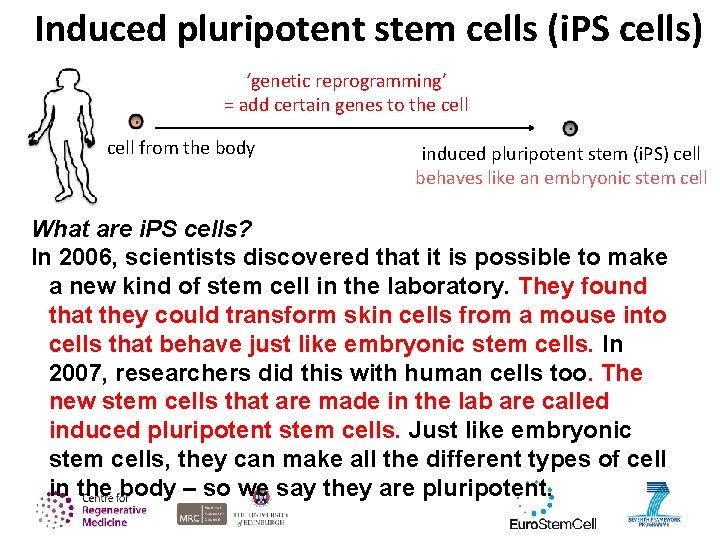
Induced pluripotent stem cells (i. PS cells) ‘genetic reprogramming’ = add certain genes to the cell from the body induced pluripotent stem (i. PS) cell behaves like an embryonic stem cell What are i. PS cells? In 2006, scientists discovered that it is possible to make a new kind of stem cell in the laboratory. They found that they could transform skin cells from a mouse into cells that behave just like embryonic stem cells. In 2007, researchers did this with human cells too. The new stem cells that are made in the lab are called induced pluripotent stem cells. Just like embryonic stem cells, they can make all the different types of cell in the body – so we say they are pluripotent.
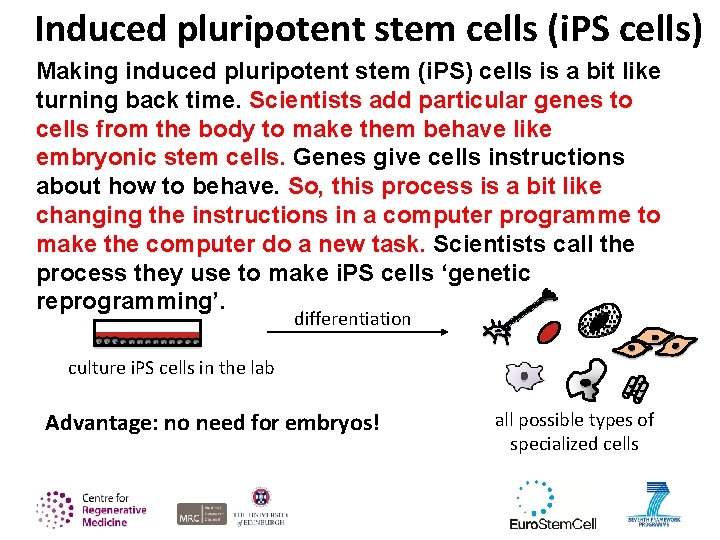
Induced pluripotent stem cells (i. PS cells) Making induced pluripotent stem (i. PS) cells is a bit like turning back time. Scientists add particular genes to cells from the body to make them behave like embryonic stem cells. Genes give cells instructions about how to behave. So, this process is a bit like changing the instructions in a computer programme to make the computer do a new task. Scientists call the process they use to make i. PS cells ‘genetic reprogramming’. differentiation culture i. PS cells in the lab Advantage: no need for embryos! all possible types of specialized cells
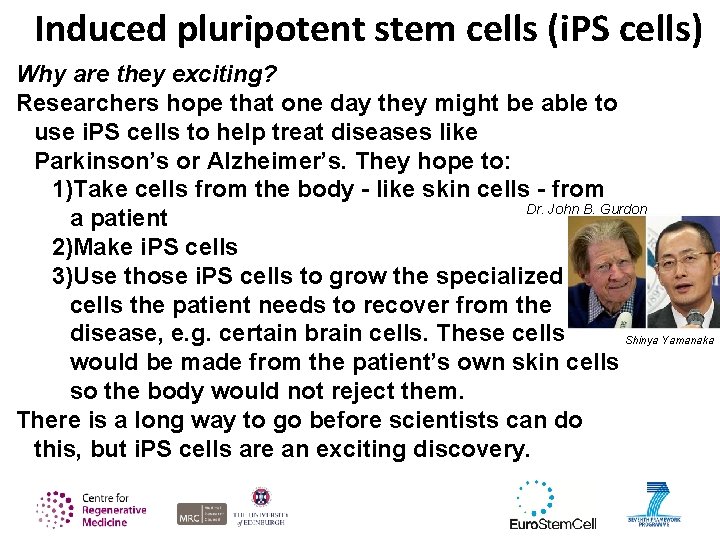
Induced pluripotent stem cells (i. PS cells) Why are they exciting? Researchers hope that one day they might be able to use i. PS cells to help treat diseases like Parkinson’s or Alzheimer’s. They hope to: 1)Take cells from the body - like skin cells - from Dr. John B. Gurdon a patient 2)Make i. PS cells 3)Use those i. PS cells to grow the specialized cells the patient needs to recover from the disease, e. g. certain brain cells. These cells Shinya Yamanaka would be made from the patient’s own skin cells so the body would not reject them. There is a long way to go before scientists can do this, but i. PS cells are an exciting discovery.
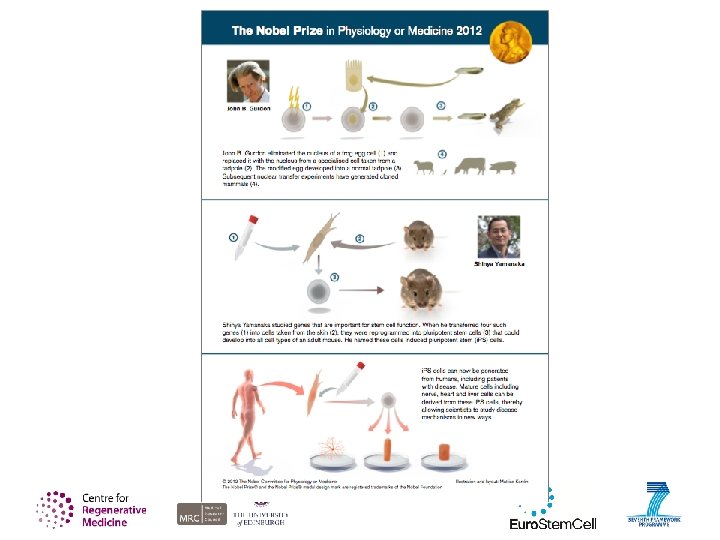

Stem cell biology in more detail
Tissue stem cell types and hierarchies
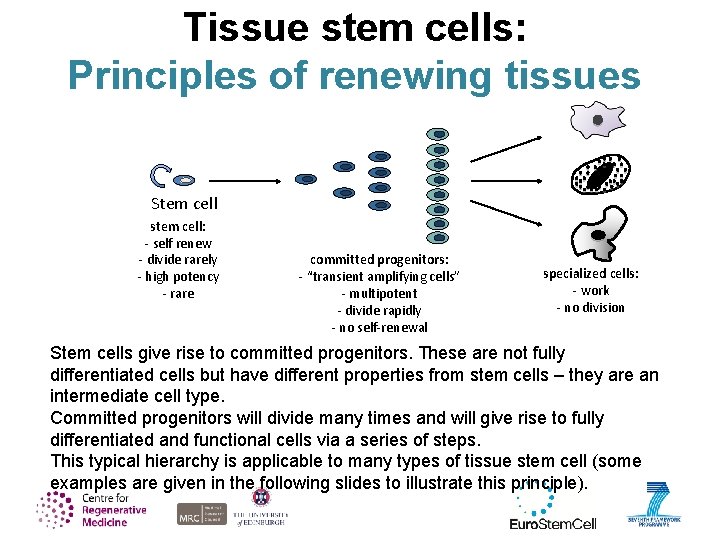
Tissue stem cells: Principles of renewing tissues Stem cell stem cell: - self renew - divide rarely - high potency - rare committed progenitors: - “transient amplifying cells” - multipotent - divide rapidly - no self-renewal specialized cells: - work - no division Stem cells give rise to committed progenitors. These are not fully differentiated cells but have different properties from stem cells – they are an intermediate cell type. Committed progenitors will divide many times and will give rise to fully differentiated and functional cells via a series of steps. This typical hierarchy is applicable to many types of tissue stem cell (some examples are given in the following slides to illustrate this principle).
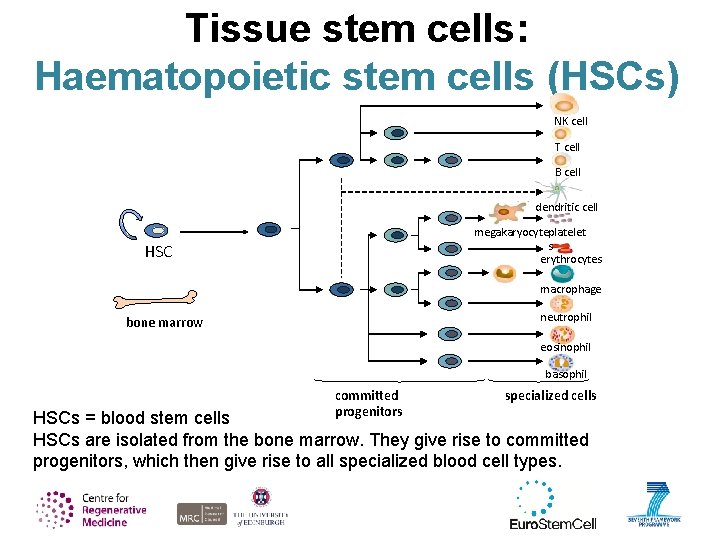
Tissue stem cells: Haematopoietic stem cells (HSCs) NK cell T cell B cell dendritic cell megakaryocyteplatelet s erythrocytes HSC macrophage neutrophil bone marrow eosinophil basophil committed progenitors specialized cells HSCs = blood stem cells HSCs are isolated from the bone marrow. They give rise to committed progenitors, which then give rise to all specialized blood cell types.
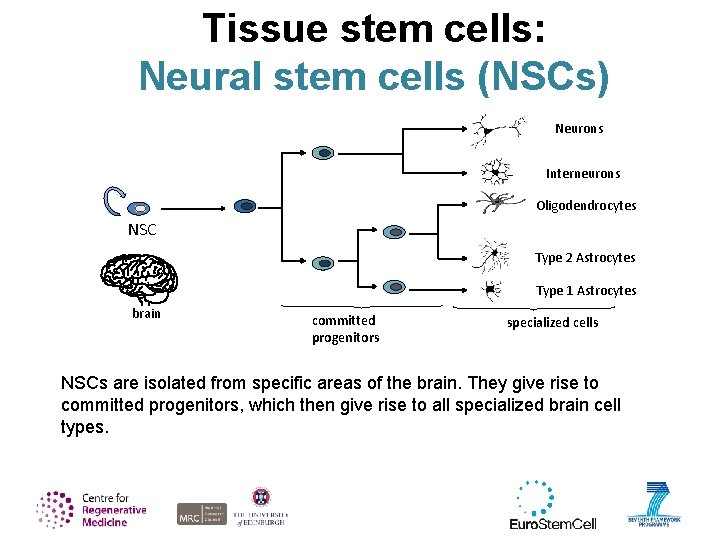
Tissue stem cells: Neural stem cells (NSCs) Neurons Interneurons Oligodendrocytes NSC Type 2 Astrocytes Type 1 Astrocytes brain committed progenitors specialized cells NSCs are isolated from specific areas of the brain. They give rise to committed progenitors, which then give rise to all specialized brain cell types.
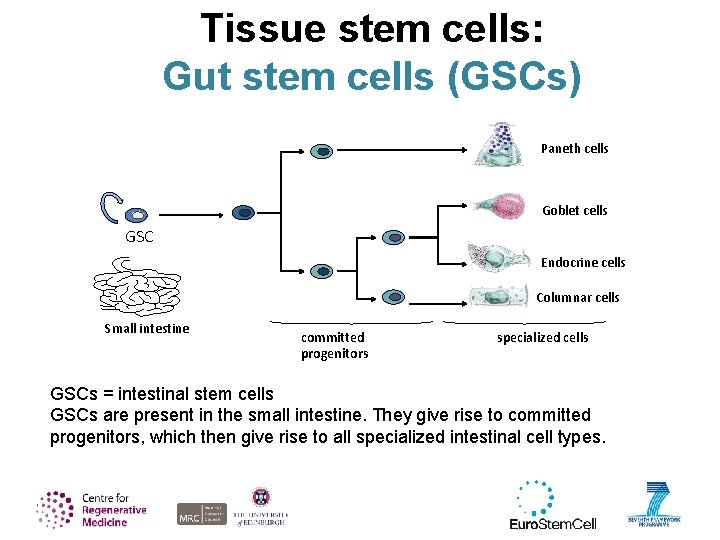
Tissue stem cells: Gut stem cells (GSCs) Paneth cells Goblet cells GSC Endocrine cells Columnar cells Small intestine committed progenitors specialized cells GSCs = intestinal stem cells GSCs are present in the small intestine. They give rise to committed progenitors, which then give rise to all specialized intestinal cell types.
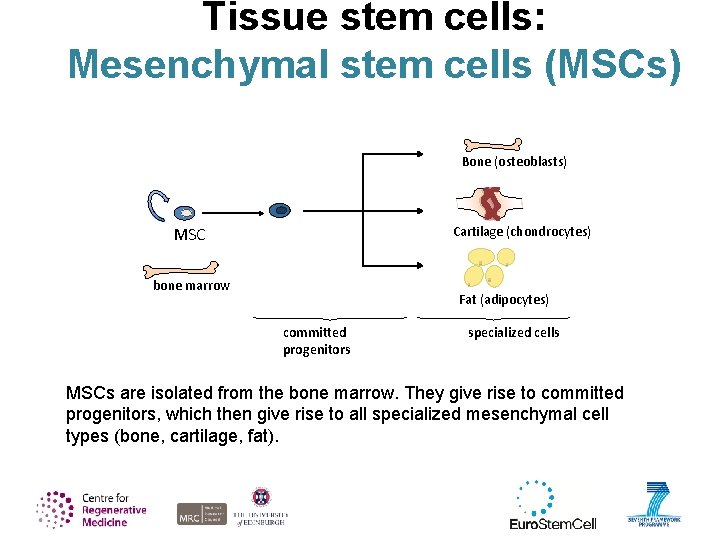
Tissue stem cells: Mesenchymal stem cells (MSCs) Bone (osteoblasts) MSC Cartilage (chondrocytes) bone marrow Fat (adipocytes) committed progenitors specialized cells MSCs are isolated from the bone marrow. They give rise to committed progenitors, which then give rise to all specialized mesenchymal cell types (bone, cartilage, fat).

What cell therapies are available right now?
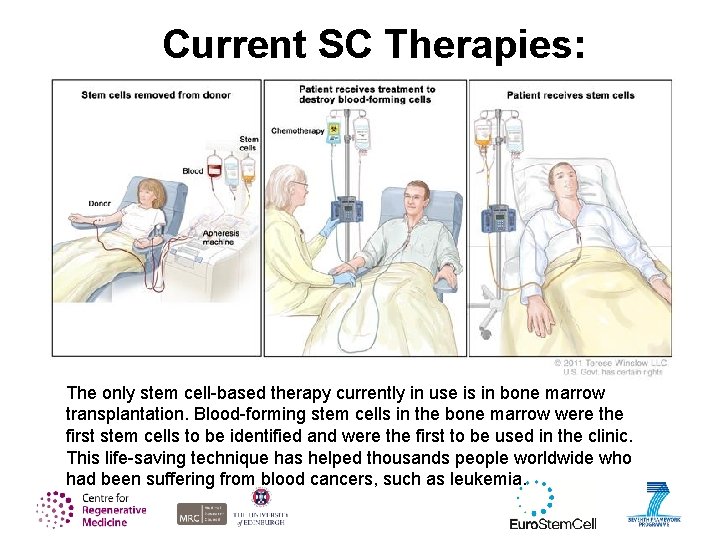
Current SC Therapies: The only stem cell-based therapy currently in use is in bone marrow transplantation. Blood-forming stem cells in the bone marrow were the first stem cells to be identified and were the first to be used in the clinic. This life-saving technique has helped thousands people worldwide who had been suffering from blood cancers, such as leukemia.
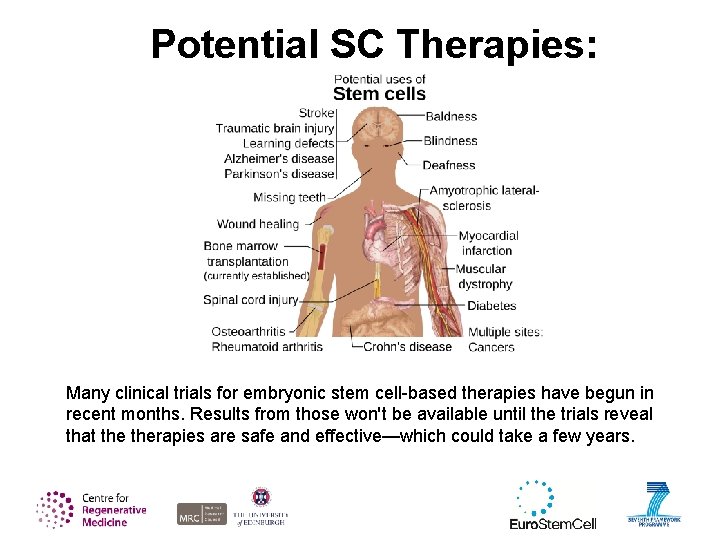
Potential SC Therapies: Many clinical trials for embryonic stem cell-based therapies have begun in recent months. Results from those won't be available until the trials reveal that therapies are safe and effective—which could take a few years.
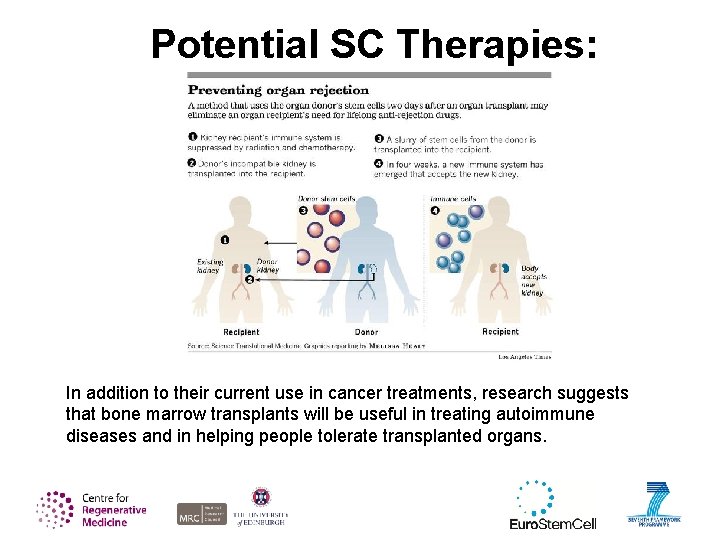
Potential SC Therapies: In addition to their current use in cancer treatments, research suggests that bone marrow transplants will be useful in treating autoimmune diseases and in helping people tolerate transplanted organs.
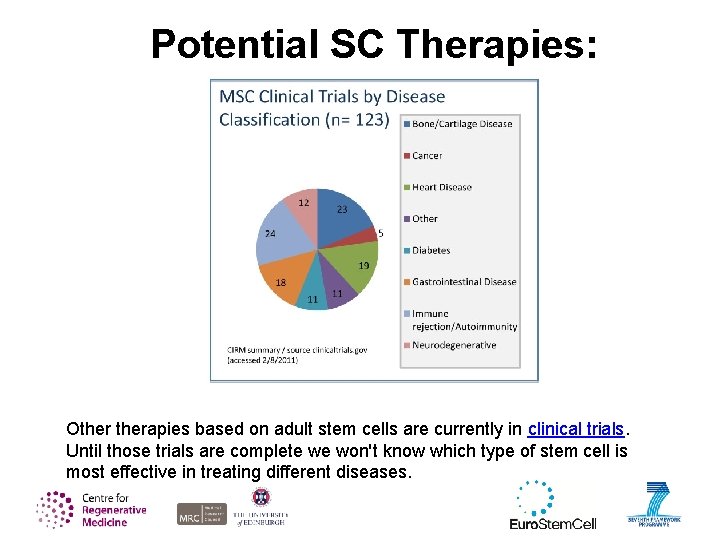
Potential SC Therapies: Otherapies based on adult stem cells are currently in clinical trials. Until those trials are complete we won't know which type of stem cell is most effective in treating different diseases.

Clinical Trials Clinical trials are conducted in a series of steps, called phases - each phase is designed to answer a separate research question. Phase I: small group testing to determine a safe dosage range, and identify side effects. Phase II: larger group testing for efficacy and to further evaluate its safety. Phase III: large group testing to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug or treatment to be used safely. Phase IV: Studies are done after the drug or treatment has been marketed to gather information on the drug's effect in various populations and any side effects associated with long-term use.

Oligodendrocyte Stem Cell Therapy TJ Atchison • Injured in a car accident in 2010 • Due to nature of injury, qualified for first ever OSC therapy • Started his own foundation • Took his first steps since the accident in 2013

Credits Picture credits Many thanks to the following people for permission to reproduce images: Slide 17, i. PS cells: Keisuke Kaji, University of Edinburgh, UK Slide 27, blood cell diagrams: Jonas Larsson, Lund Univeristy, Sweden Slide 29, intestinal cell diagrams: Hans Clevers and Nick Barker, Hubrecht Institute, The Netherlands Should you wish to re-use any of the images listed above, please contact the owner. All other images in this presentation can be re-used freely. Acknowledgements Particular thanks to Dr Christele Gonneau for creating these slides and working tirelessly to help ensure the notes are correct. Thanks also to Freddy Radtke of EPFL, Switzerland, whose slide we copied to make slide 27 on tissue stem cells.
- Slides: 53