Intro to Organic Chemistry Organic Chemistry Introduction Organic





- Slides: 27

Intro to Organic Chemistry

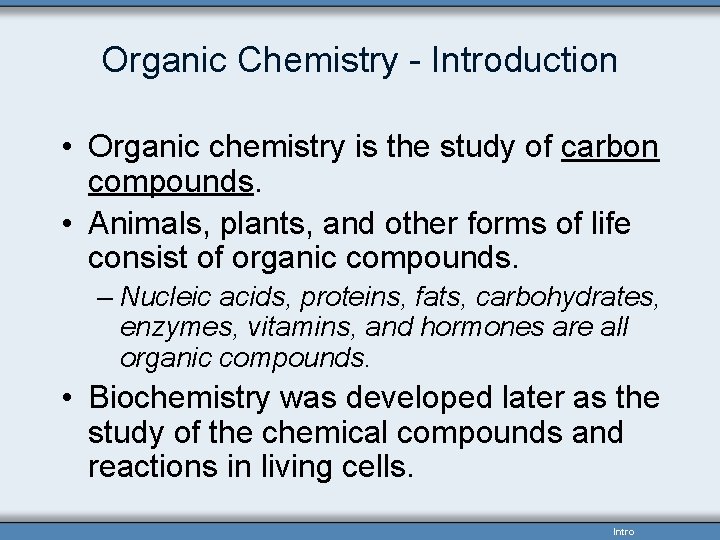
Organic Chemistry - Introduction • Organic chemistry is the study of carbon compounds. • Animals, plants, and other forms of life consist of organic compounds. – Nucleic acids, proteins, fats, carbohydrates, enzymes, vitamins, and hormones are all organic compounds. • Biochemistry was developed later as the study of the chemical compounds and reactions in living cells. Intro

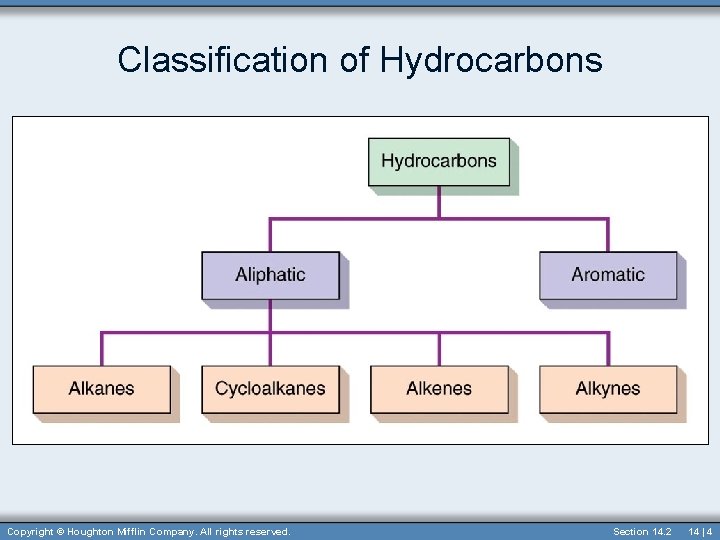
Hydrocarbons • Simplest organic compound • Contain only carbon and hydrogen • Hydrocarbons can be divided into aromatic and aliphatic hydrocarbons. 14 | 3

Classification of Hydrocarbons Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 2 14 | 4

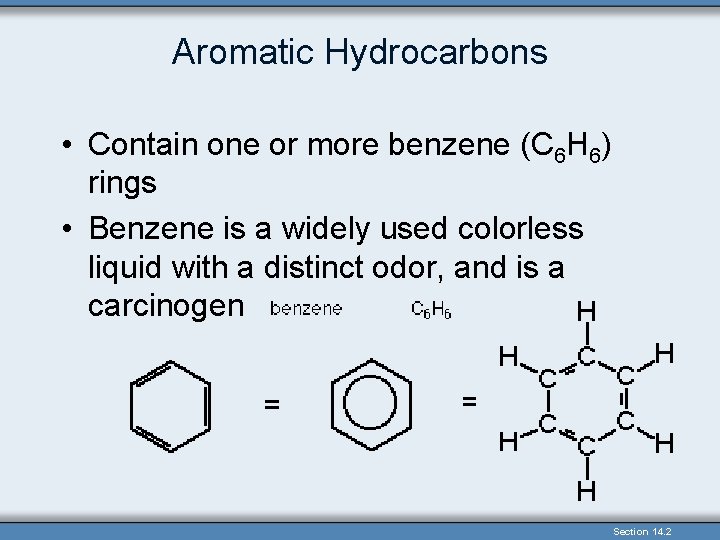
Aromatic Hydrocarbons • Contain one or more benzene (C 6 H 6) rings • Benzene is a widely used colorless liquid with a distinct odor, and is a carcinogen Section 14. 2

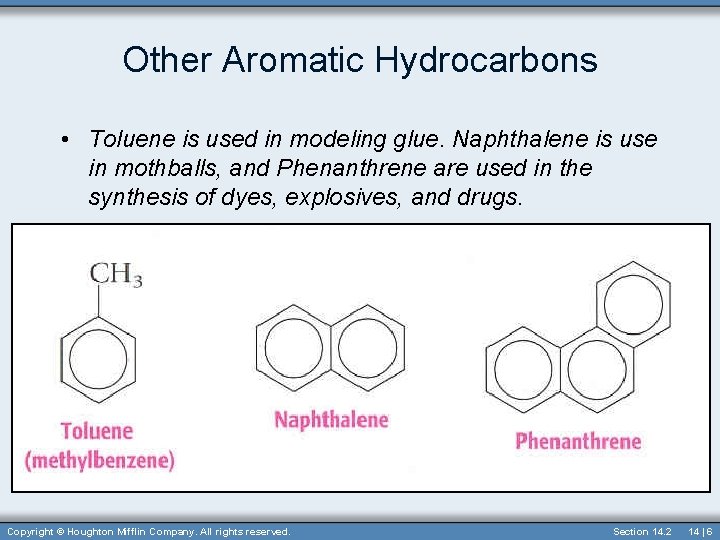
Other Aromatic Hydrocarbons • Toluene is used in modeling glue. Naphthalene is use in mothballs, and Phenanthrene are used in the synthesis of dyes, explosives, and drugs. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 2 14 | 6

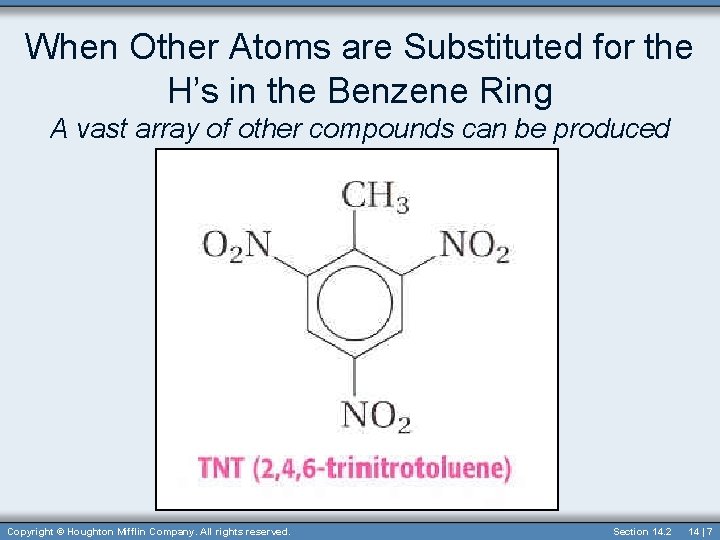
When Other Atoms are Substituted for the H’s in the Benzene Ring A vast array of other compounds can be produced Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 2 14 | 7

Aliphatic Hydrocarbons • Aliphatic hydrocarbons have no benzene rings. • Divided into four major divisions: – Alkanes – Cycloalkanes – ring shaped – Alkenes – contain at least one double bond between carbons – Alkynes – contain at least one tripple bond between carbons Section 14. 3

Alkanes • Alkanes are hydrocarbons that contain only single bonds. • Alkanes are said to be saturated hydrocarbons – Because their hydrogen content is at a maximum. • Alkane general formula Cn. H 2 n + 2 • The names of alkanes all end in “-ane. ” – – Methane – CH 4 Ethane – C 2 H 6 Propane – C 3 H 8 Butane – C 4 H 10 Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 9

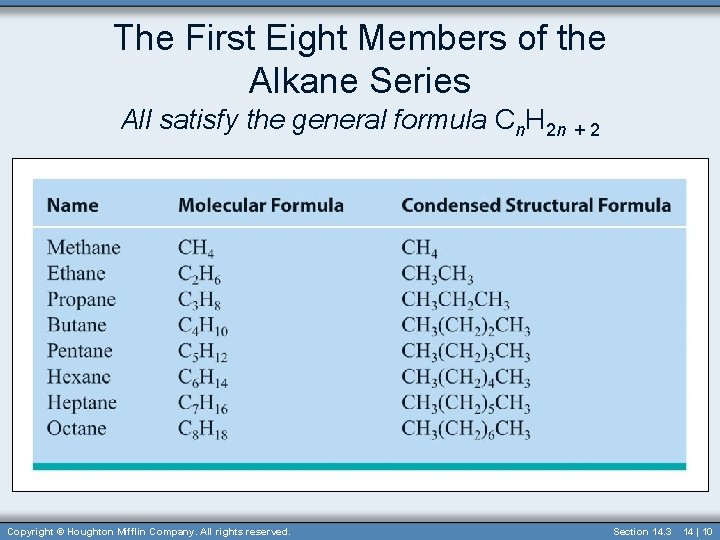
The First Eight Members of the Alkane Series All satisfy the general formula Cn. H 2 n + 2 Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 10

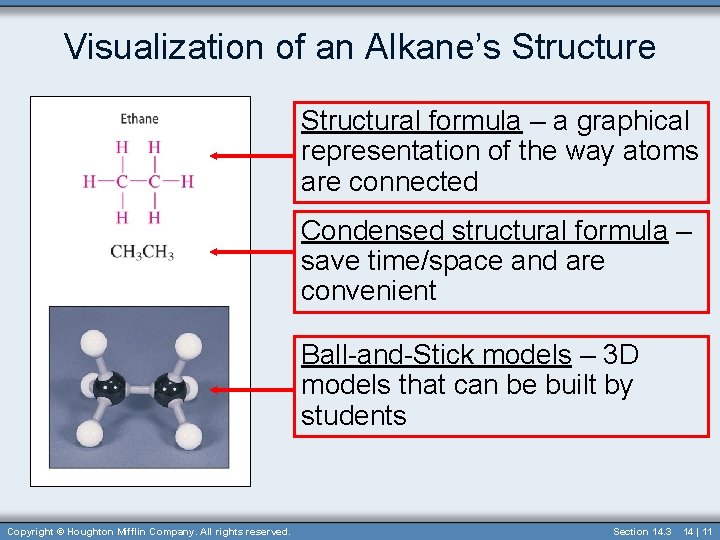
Visualization of an Alkane’s Structure Structural formula – a graphical representation of the way atoms are connected Condensed structural formula – save time/space and are convenient Ball-and-Stick models – 3 D models that can be built by students Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 11

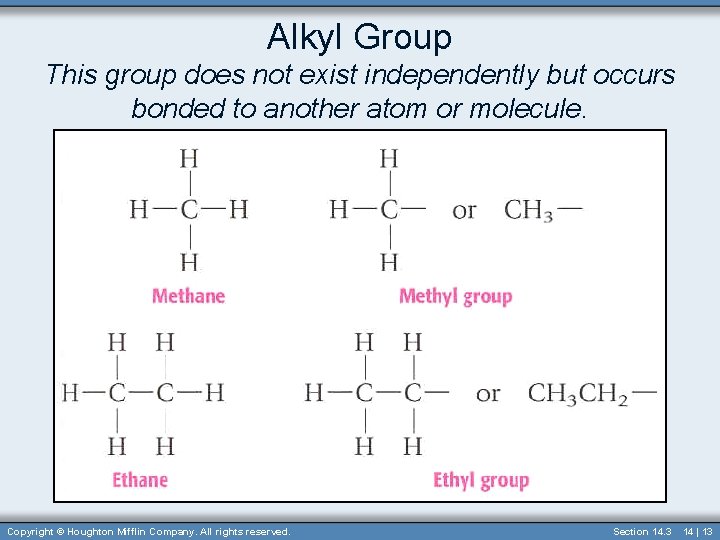
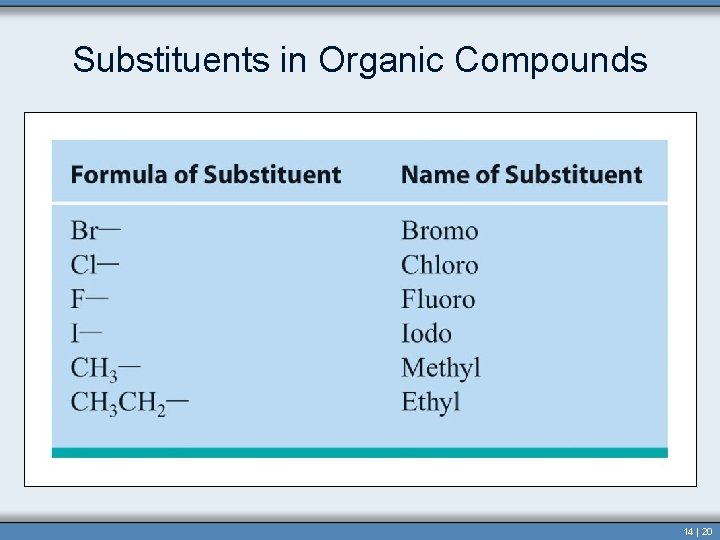
Alkyl Group • Alkyl group contains one less hydrogen than the corresponding alkane. • In naming this group the “-ane” is dropped and “-yl” is added. – Methyl– Ethyl– Propyl– Butyl- • Used as prefixes when naming structures 14 | 12

Alkyl Group This group does not exist independently but occurs bonded to another atom or molecule. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 13

Constitutional Isomers • Compounds that have the same molecular formula but different structural formulas • In the case of many alkanes there is more than one way to arrange the atoms Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 14

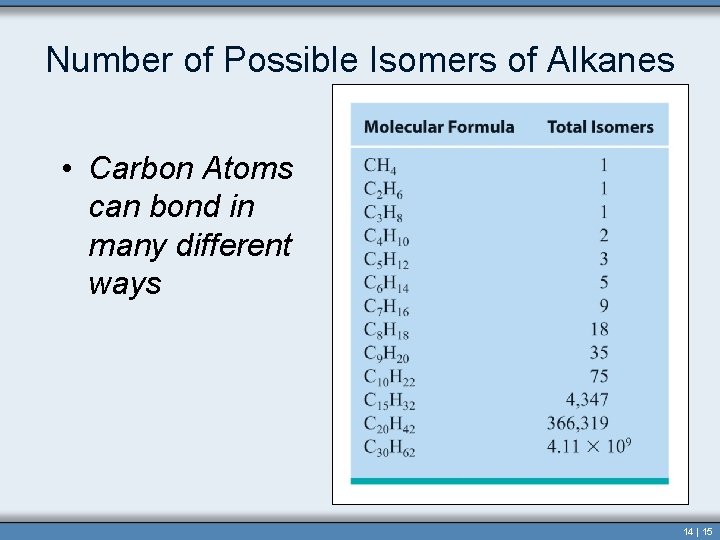
Number of Possible Isomers of Alkanes • Carbon Atoms can bond in many different ways 14 | 15

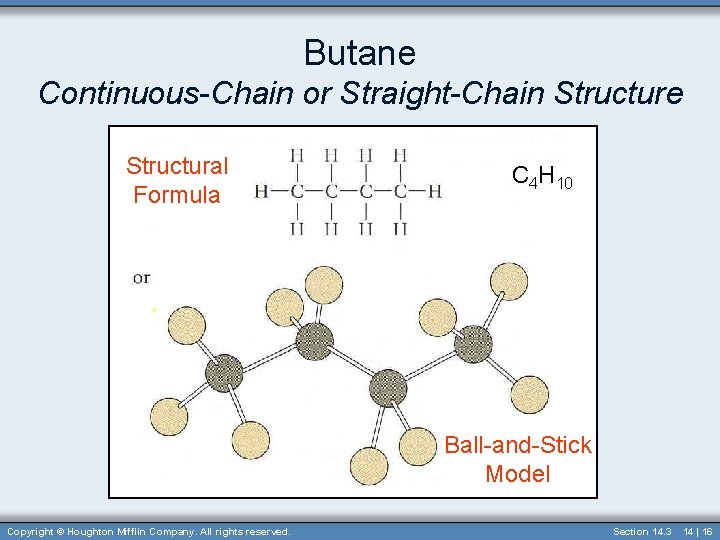
Butane Continuous-Chain or Straight-Chain Structure Structural Formula C 4 H 10 Ball-and-Stick Model Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 16

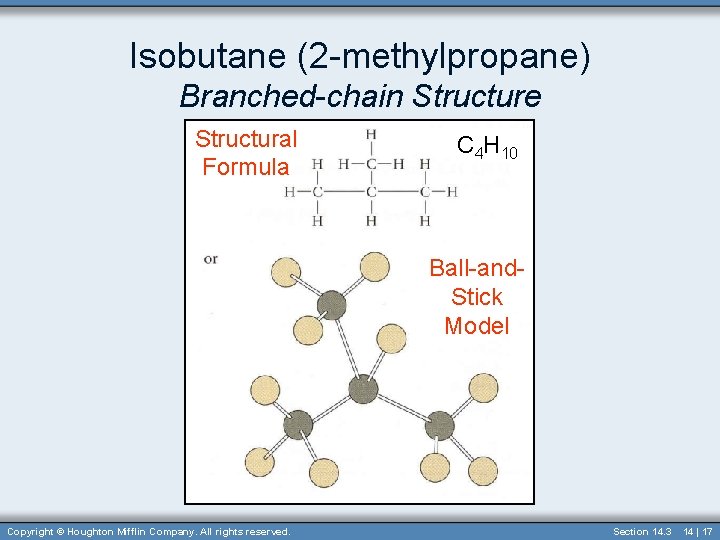
Isobutane (2 -methylpropane) Branched-chain Structure Structural Formula C 4 H 10 Ball-and. Stick Model Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 17

IUPAC System of Nomenclature For Alkanes • Compound is named as a derivative of the longest continuous chain of C atoms. • The C atoms on the main chain are numbered by counting from the end of the chain nearest the substituents. – Each substituent must have a number. • Positions & names of the substituents added – If necessary, substituents named alphabetically – More than one of same type substituent – di, tri, tetra Section 14. 3

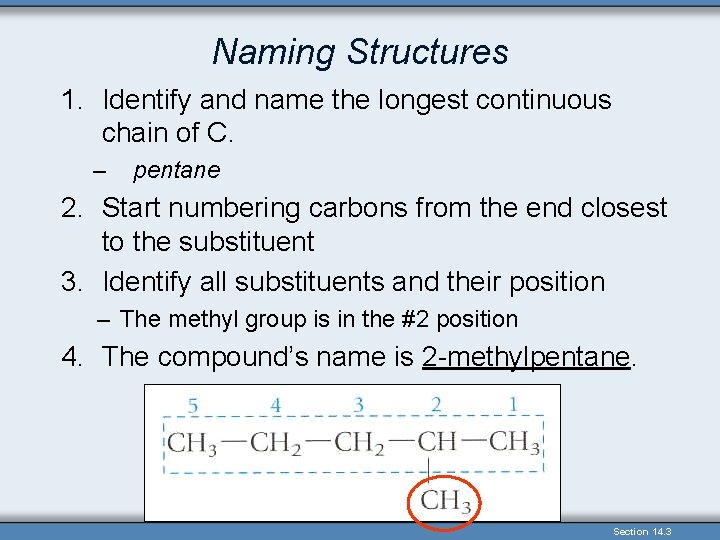
Naming Structures 1. Identify and name the longest continuous chain of C. – pentane 2. Start numbering carbons from the end closest to the substituent 3. Identify all substituents and their position – The methyl group is in the #2 position 4. The compound’s name is 2 -methylpentane. Section 14. 3

Substituents in Organic Compounds 14 | 20

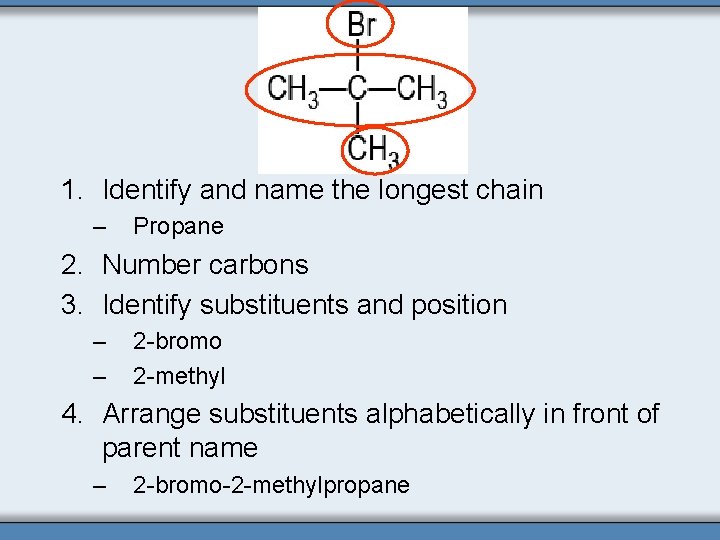
1. Identify and name the longest chain – Propane 2. Number carbons 3. Identify substituents and position – – 2 -bromo 2 -methyl 4. Arrange substituents alphabetically in front of parent name – 2 -bromo-2 -methylpropane

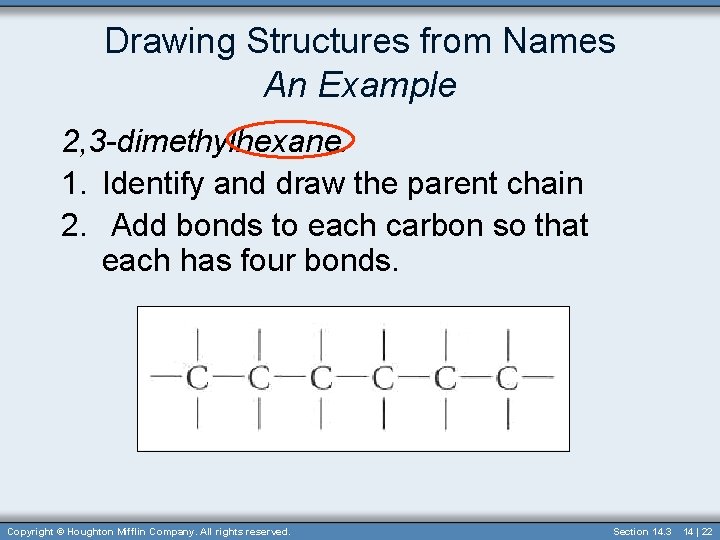
Drawing Structures from Names An Example 2, 3 -dimethylhexane. 1. Identify and draw the parent chain 2. Add bonds to each carbon so that each has four bonds. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 22

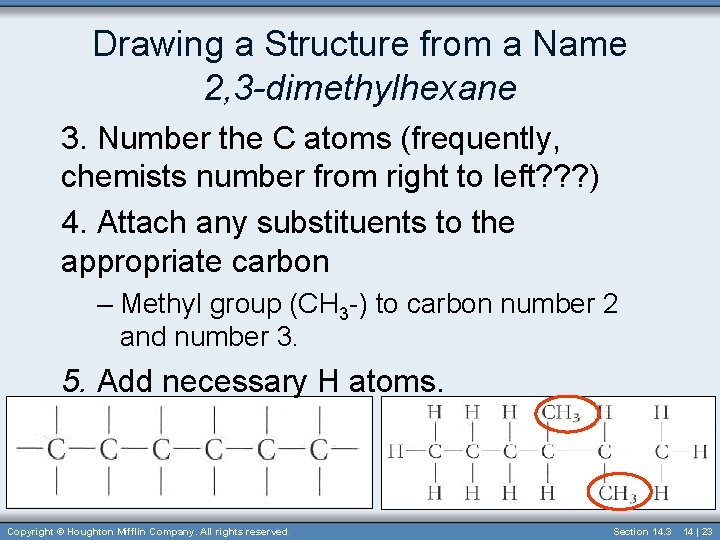
Drawing a Structure from a Name 2, 3 -dimethylhexane 3. Number the C atoms (frequently, chemists number from right to left? ? ? ) 4. Attach any substituents to the appropriate carbon – Methyl group (CH 3 -) to carbon number 2 and number 3. 5. Add necessary H atoms. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 23

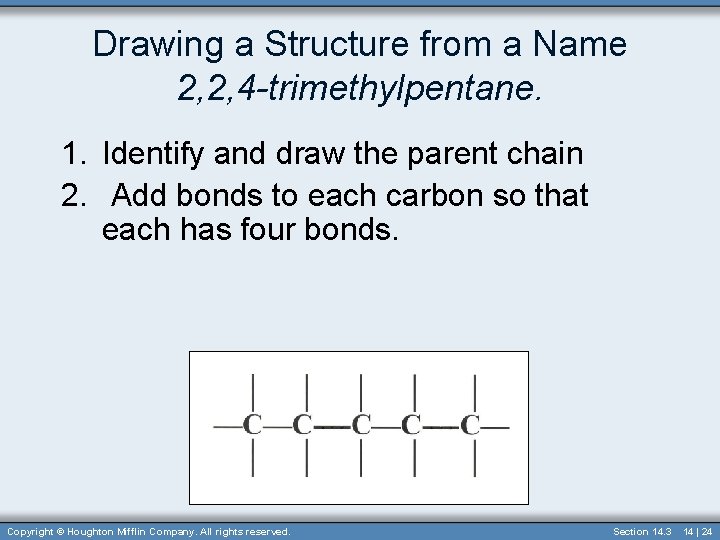
Drawing a Structure from a Name 2, 2, 4 -trimethylpentane. 1. Identify and draw the parent chain 2. Add bonds to each carbon so that each has four bonds. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 24

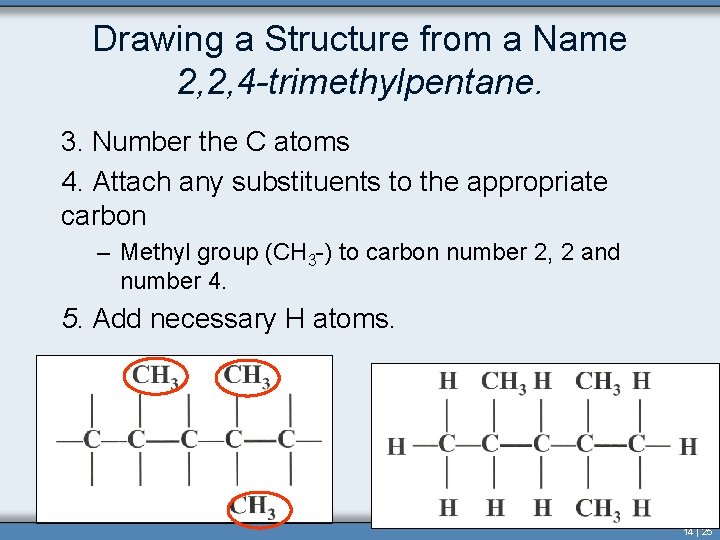
Drawing a Structure from a Name 2, 2, 4 -trimethylpentane. 3. Number the C atoms 4. Attach any substituents to the appropriate carbon – Methyl group (CH 3 -) to carbon number 2, 2 and number 4. 5. Add necessary H atoms. 14 | 25

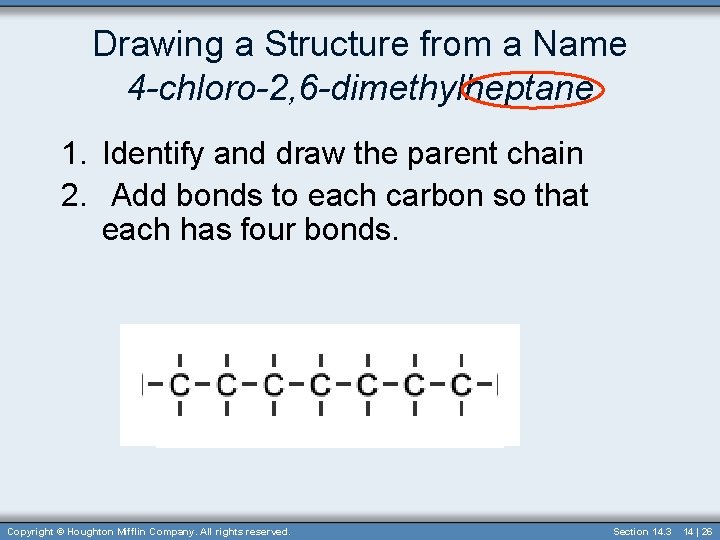
Drawing a Structure from a Name 4 -chloro-2, 6 -dimethylheptane 1. Identify and draw the parent chain 2. Add bonds to each carbon so that each has four bonds. Copyright © Houghton Mifflin Company. All rights reserved. Section 14. 3 14 | 26

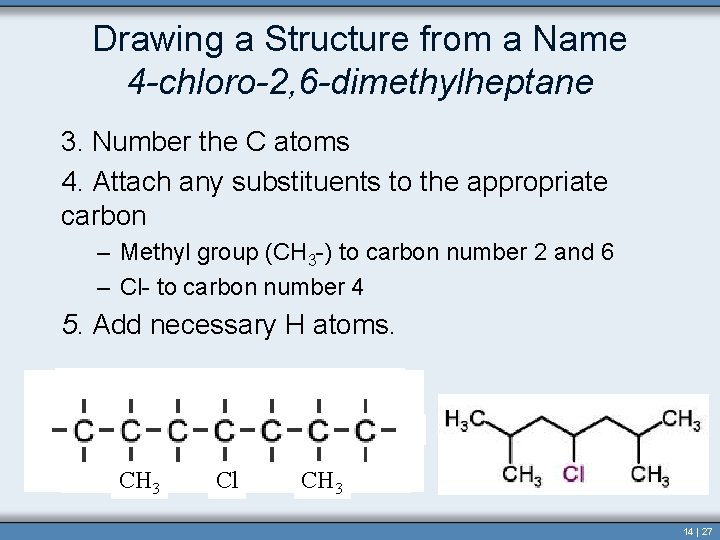
Drawing a Structure from a Name 4 -chloro-2, 6 -dimethylheptane 3. Number the C atoms 4. Attach any substituents to the appropriate carbon – Methyl group (CH 3 -) to carbon number 2 and 6 – Cl- to carbon number 4 5. Add necessary H atoms. CH 3 Cl CH 3 14 | 27