Intro to Molecular Compounds l l Molecule smallest






- Slides: 14

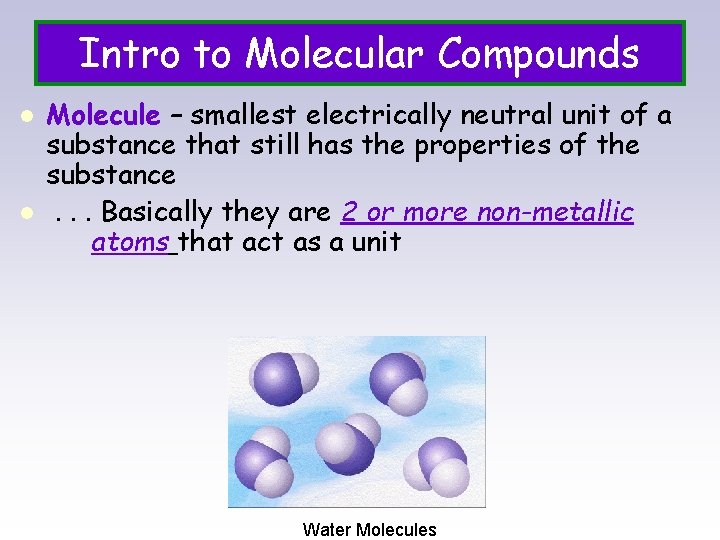
Intro to Molecular Compounds l l Molecule – smallest electrically neutral unit of a substance that still has the properties of the substance. . . Basically they are 2 or more non-metallic atoms that act as a unit Water Molecules

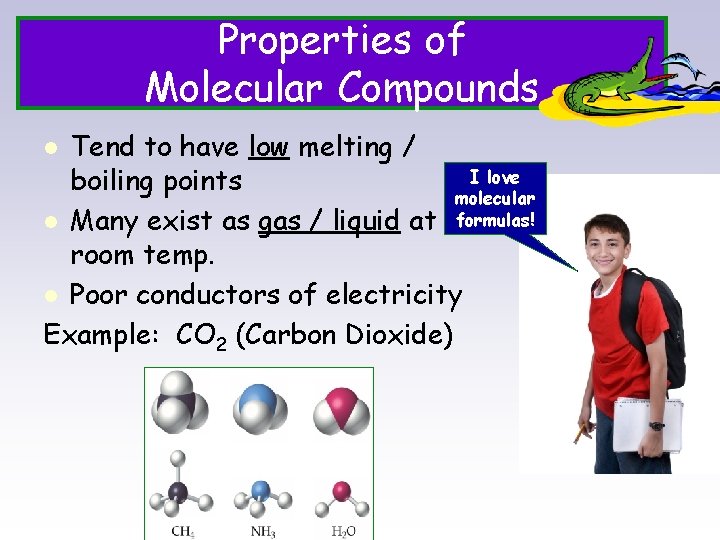
Properties of Molecular Compounds Tend to have low melting / I love boiling points molecular l Many exist as gas / liquid at formulas! room temp. l Poor conductors of electricity Example: CO 2 (Carbon Dioxide) l

Binary Molecular Compounds – Compounds composed of two nonmetallic elements. Te At

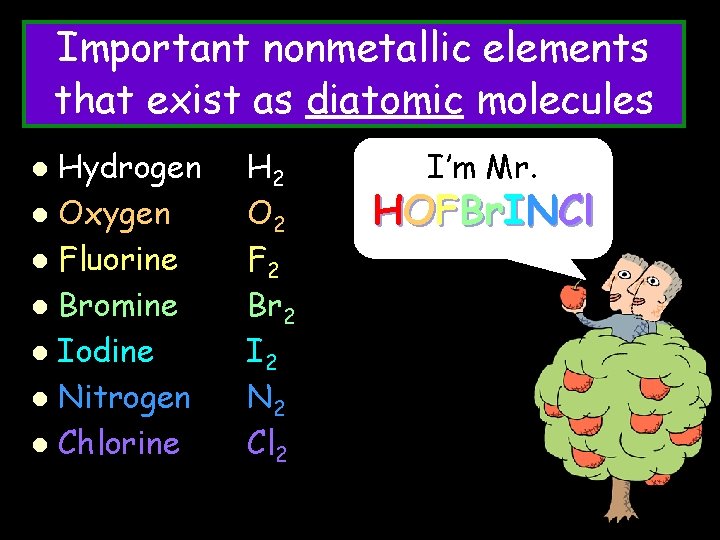
Important nonmetallic elements that exist as diatomic molecules Hydrogen l Oxygen l Fluorine l Bromine l Iodine l Nitrogen l Chlorine l H 2 O 2 F 2 Br 2 I 2 N 2 Cl 2 I’m Mr. HOFBr. INCl

Naming Binary Molecular Compounds

Why is a naming system important? Carbon and oxygen combine to form carbon monoxide (CO) and carbon dioxide (CO 2), but these two invisible gases are very different. Sitting in a room with small amounts of CO 2 in the air would not present any problems. If the same amount of CO were in the room, you could die of asphyxiation. A naming system that distinguishes between compounds is needed.

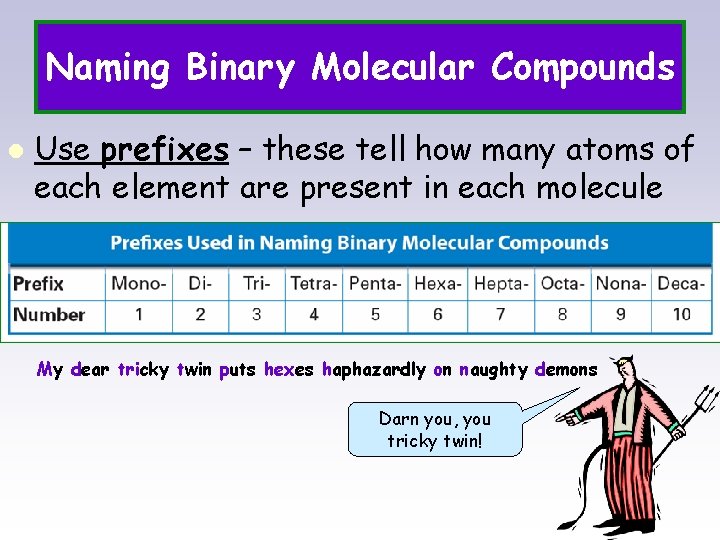
Naming Binary Molecular Compounds l Use prefixes – these tell how many atoms of each element are present in each molecule My dear tricky twin puts hexes haphazardly on naughty demons Darn you, you tricky twin!

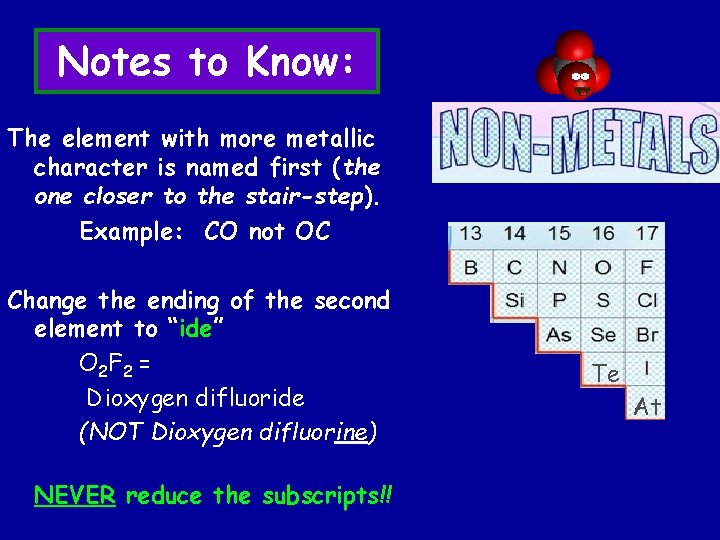
Notes to Know: The element with more metallic character is named first (the one closer to the stair-step). Example: CO not OC Change the ending of the second element to “ide” O 2 F 2 = Dioxygen difluoride (NOT Dioxygen difluorine) NEVER reduce the subscripts!! Te At

Notes to know: Do not write “mono” if there is only 1 of the first element CO 2 = Carbon dioxide (NOT Monocarbon dioxide) SF 2 = Sulfur difluoride (NOT Monosulfur difluoride) Did I mention. . . NEVER, EVER reduce the subscripts of molecules!!!

For those who care about spelling. . . When a prefix is added that places two vowels next to each other, the ‘a’ or ‘o’ on the prefix is dropped. The ‘i’ in ‘di-’ or ‘tri’ is never dropped. CO = carbon monoxide NOT carbon monooxide Br 2 O 8 = dibromine octoxide BI 3 = NOT dibromine octaoxide boron triiodide NOT boron triodide

Practice Write the correct name/formula for: 4) PCl 5 phosphorus pentachloride S 2 Br 6 disulfur hexabromide BN boron mononitride disulfur dichloride S 2 Cl 2 5) silicon tetrachloride Si. Cl 4 1) 2) 3) Oh yeah, just one more thing. . . DON’T DO IT!!! Don’t reduce the subscripts!!!

Practice 1. Which of the following compounds is named INCORRECTLY? A. CS 2, carbon disulfide B. BCl 3, boron trichloride C. IF 7, iodine heptafluoride D. PCl 5, phosphorus hexachloride

Practice 2. Which of the following molecular compounds is named INCORRECTLY? A. Sb. Cl 3, antimony trichloride B. C 2 O 5, dicarbon pentoxide C. CF 4, carbon tetrafluoride D. H 3 As, hydrogen arsenide

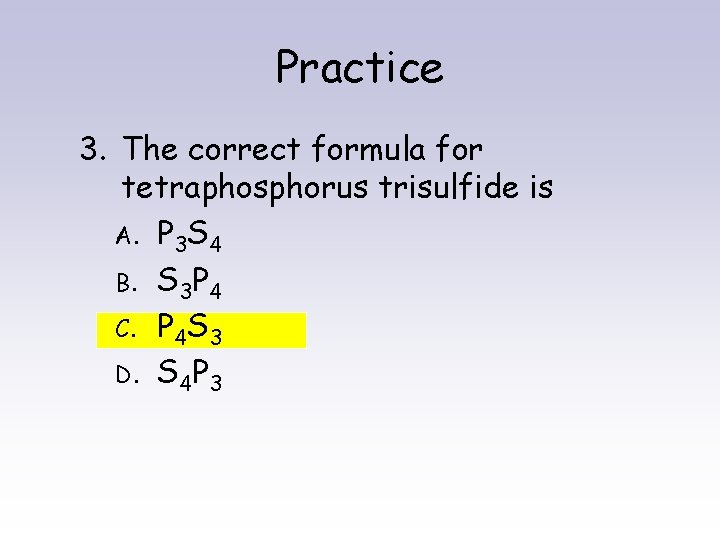
Practice 3. The correct formula for tetraphosphorus trisulfide is A. P 3 S 4 B. S 3 P 4 C. P 4 S 3 D. S 4 P 3