Intro to Diffusion Drill Would you rather stand

![Slide 9 Recap Diffusion • Molecules move from [high] to [low] • Equilibrium is Slide 9 Recap Diffusion • Molecules move from [high] to [low] • Equilibrium is](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-10.jpg)

![Slide 11 Osmosis: Water Movement • Diffusion occurs from an area of high [water] Slide 11 Osmosis: Water Movement • Diffusion occurs from an area of high [water]](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-12.jpg)
![Slide 12 Direction of Osmosis • Hypertonic – [Solute] outside the cell is higher Slide 12 Direction of Osmosis • Hypertonic – [Solute] outside the cell is higher](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-13.jpg)
![Slide 13 Direction of Osmosis • Hypotonic – [Solute] inside the cell is higher Slide 13 Direction of Osmosis • Hypotonic – [Solute] inside the cell is higher](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-14.jpg)
![Slide 14 Direction of Osmosis • Isotonic – [Solute] inside and outside the cell Slide 14 Direction of Osmosis • Isotonic – [Solute] inside and outside the cell](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-15.jpg)
![Slide 15 Recap Osmosis • Water molecules move from [high] to [low] • Hypertonic: Slide 15 Recap Osmosis • Water molecules move from [high] to [low] • Hypertonic:](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-16.jpg)
- Slides: 16

Intro to Diffusion Drill: Would you rather stand in a very crowded room, which made it hard to move through, or in a room that was less crowded, which made it easy to move through? Briefly explain. Outcome: Explain how diffusion works. Discuss the purpose of diffusion in the body. Draw the movement of water/salt due to diffusion.

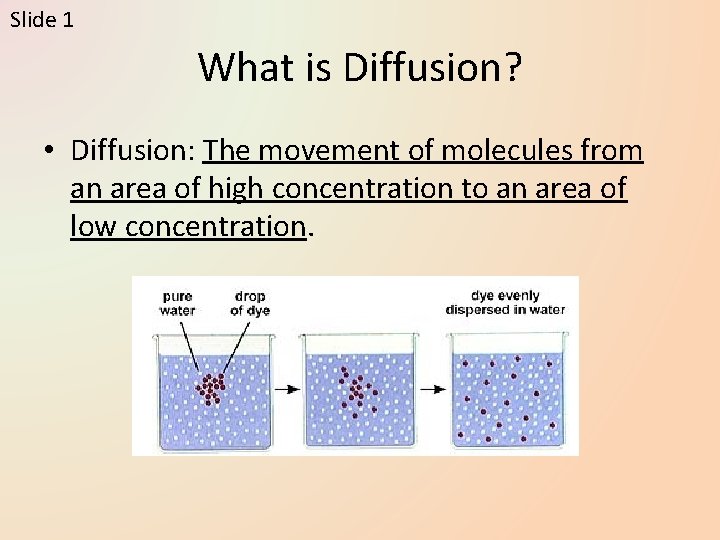
Slide 1 What is Diffusion? • Diffusion: The movement of molecules from an area of high concentration to an area of low concentration.

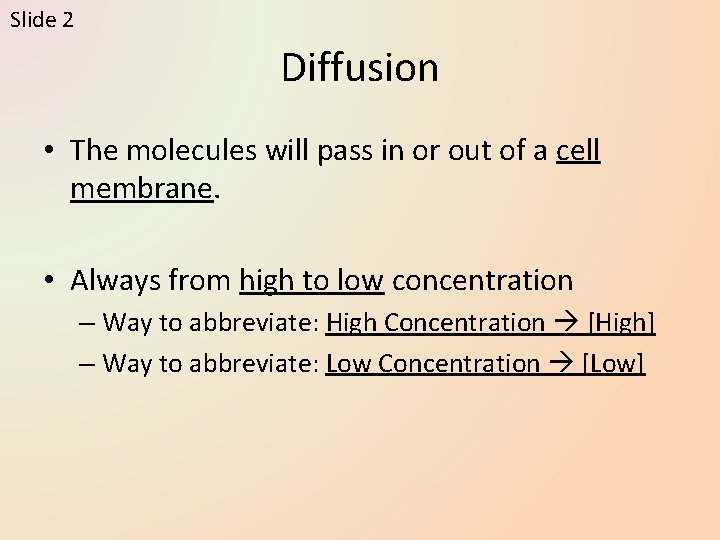
Slide 2 Diffusion • The molecules will pass in or out of a cell membrane. • Always from high to low concentration – Way to abbreviate: High Concentration [High] – Way to abbreviate: Low Concentration [Low]

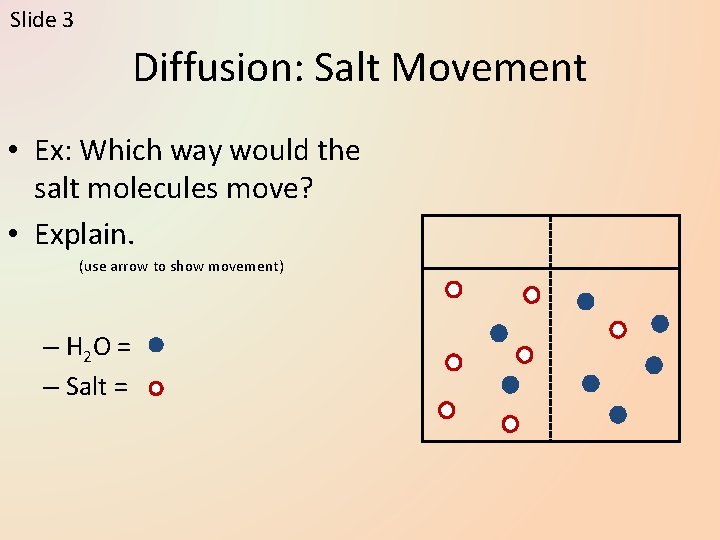
Slide 3 Diffusion: Salt Movement • Ex: Which way would the salt molecules move? • Explain. (use arrow to show movement) – H 2 O = – Salt =

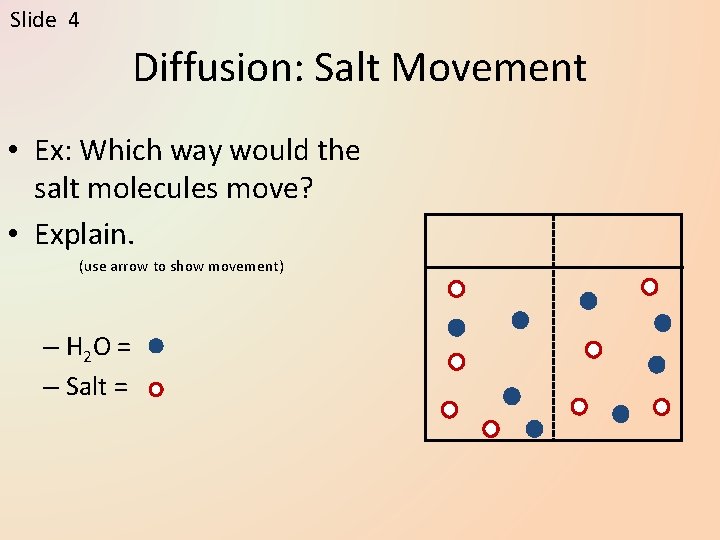
Slide 4 Diffusion: Salt Movement • Ex: Which way would the salt molecules move? • Explain. (use arrow to show movement) – H 2 O = – Salt =

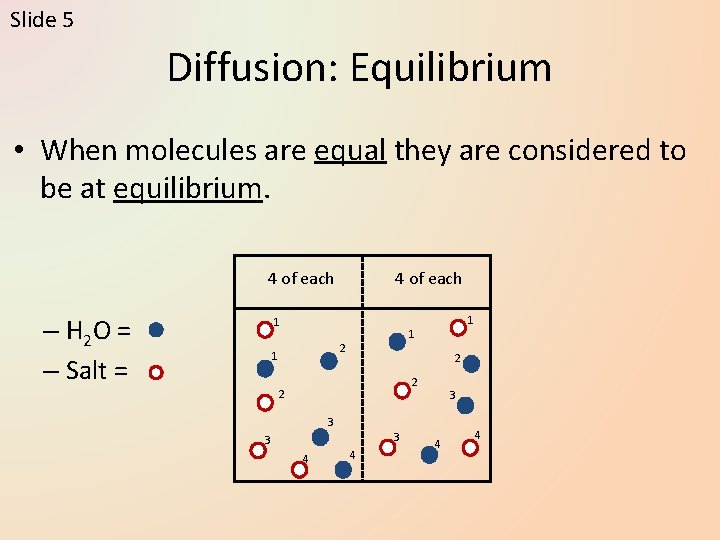
Slide 5 Diffusion: Equilibrium • When molecules are equal they are considered to be at equilibrium. 4 of each – H 2 O = – Salt = 4 of each 1 2 1 1 1 2 2 2 3 3 4 4

Slide 6 Diffusion Movement • Kinetic E: The movement of molecules being diffused across a membrane. • Kinetic E keeps gas/liquid molecules in constant motion causing them to randomly move away from each other. • The rate (speed) of this movement is determined by the temperature and the size of molecules.

Slide 7 Diffusion Movement • Temperature – Increasing the temp. • Molecules diffuse faster – Decreasing the temp. • Molecules diffuse slower

Slide 8 Diffusion Movement • Size of molecules - Smaller molecules • Molecules diffuse faster - Bigger molecules • Molecules diffuse slower
![Slide 9 Recap Diffusion Molecules move from high to low Equilibrium is Slide 9 Recap Diffusion • Molecules move from [high] to [low] • Equilibrium is](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-10.jpg)
Slide 9 Recap Diffusion • Molecules move from [high] to [low] • Equilibrium is when all particles on both sides are equal in number • Kinetic E causes them to move randomly • Temperature and Size changes rate of movement

Slide 10 Osmosis • Osmosis: The movement of water across a semipermeable membrane. • The most important thing here is that: – We are ONLY talking about the movement of WATER
![Slide 11 Osmosis Water Movement Diffusion occurs from an area of high water Slide 11 Osmosis: Water Movement • Diffusion occurs from an area of high [water]](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-12.jpg)
Slide 11 Osmosis: Water Movement • Diffusion occurs from an area of high [water] (less solute) to an area of lower [water] (more solute) • Explain which way the water molecules will move. – H 2 O: – Salt:
![Slide 12 Direction of Osmosis Hypertonic Solute outside the cell is higher Slide 12 Direction of Osmosis • Hypertonic – [Solute] outside the cell is higher](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-13.jpg)
Slide 12 Direction of Osmosis • Hypertonic – [Solute] outside the cell is higher (less water) – Water moves out of the cell – Cell shrinks
![Slide 13 Direction of Osmosis Hypotonic Solute inside the cell is higher Slide 13 Direction of Osmosis • Hypotonic – [Solute] inside the cell is higher](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-14.jpg)
Slide 13 Direction of Osmosis • Hypotonic – [Solute] inside the cell is higher (less water) – Water moves in of the cell – Cell grows and can burst
![Slide 14 Direction of Osmosis Isotonic Solute inside and outside the cell Slide 14 Direction of Osmosis • Isotonic – [Solute] inside and outside the cell](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-15.jpg)
Slide 14 Direction of Osmosis • Isotonic – [Solute] inside and outside the cell is equal – Water moves in and out of the cell equally – Cell stays the same
![Slide 15 Recap Osmosis Water molecules move from high to low Hypertonic Slide 15 Recap Osmosis • Water molecules move from [high] to [low] • Hypertonic:](https://slidetodoc.com/presentation_image/7fad4c7b1469a87bd92e5917f82c2850/image-16.jpg)
Slide 15 Recap Osmosis • Water molecules move from [high] to [low] • Hypertonic: • Hypotonic: • Isotonic: