Intro to Chemistry Chem 1020 Lab Acids Bases

Intro to Chemistry Chem 1020 Lab Acids, Bases, and Indicators Chemistry Department Minneapolis Community & Technical College 1

Overview • Part I Concept review of p. H • Part II Indicator • Part III p. H standard solutions • Part IV Household products • Part V p. H paper 2

Part I. p. H • p. H is used to indicate the acidity of a solution. • The lower the p. H value, the more acidic the solution is. • Solutions can be classified based on their p. H values: p. H < 7. 0, the solution is acidic p. H = 7. 0, the solution is neutral p. H > 7. 0, the solution is basic The goal of this experiment is to determine if each of the 12 given household products is acidic, neutral, or basic after measuring their individual p. H values. 3

Part II. Indicator There are many different ways of determining p. H values of solutions. One easy way is to use an indicator. Indicator is a substance that changes its color based on the p. H of its environment. A substance present in the juice from this lovely red cabbage will be used as an indicator in this experiment. 4

Shredded red cabbage leaves are heated in distilled water in advance. The filtered cabbage juice is stored in a separatory funnel for dispense. Obtain 40 m. L of juice. Now it seems that you are ready to explore with this wonderful indicator juice! But wait! How do you know what color of the indicator is associated with what p. H value? So before you work on the household products, you need to test the red cabbage indicator on a series of standard solutions with known p. H values. 5
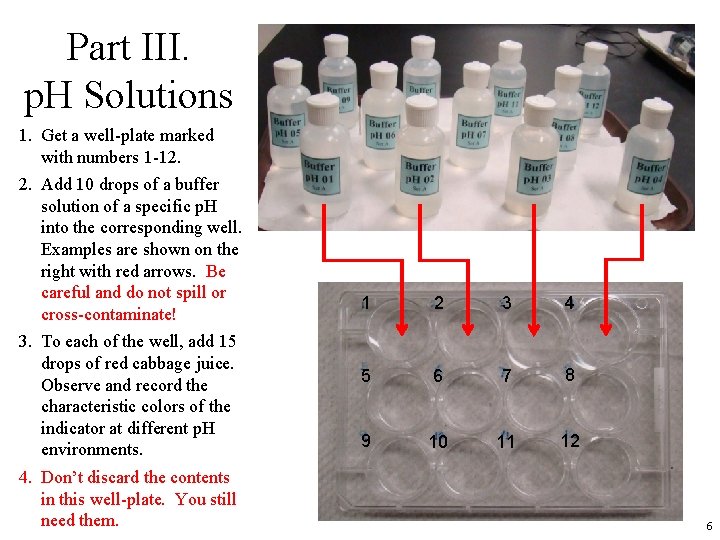
Part III. p. H Solutions 1. Get a well-plate marked with numbers 1 -12. 2. Add 10 drops of a buffer solution of a specific p. H into the corresponding well. Examples are shown on the right with red arrows. Be careful and do not spill or cross-contaminate! 3. To each of the well, add 15 drops of red cabbage juice. Observe and record the characteristic colors of the indicator at different p. H environments. 4. Don’t discard the contents in this well-plate. You still need them. 1 2 3 4 5 6 7 8 9 10 11 12 6

Part IV. Household Products 1. Get a different well-plate marked with letters A-L. 2. To each of the well, add 15 drops of red cabbage juice. 3. Add a certain household product into the corresponding well: 2 drops if it is a liquid and a match-head amount if solid. An example is shown below with the red arrow. Be careful and do not spill or cross-contaminate! 4. Compare the color in a specific well with those of 12 wells in the well-plate labeled with numbers (see the previous slide). For example, if the color in well “A” is somewhere in between the colors of wells “ 3” and “ 4”, record the p. H of vinegar as 3. 5. Household product A, vinegar, goes into well “A”. 7

Part V. What if…. . a color in the well of a household product does not match any one of those 12 colors of the p. H standard solutions? ! • We will have to use p. H paper which is coated with a different indicator. The correlation between the different colors of this indicator and the p. H values can be easily found on the cover of the container. • Use a pair of tweezers to pick up the p. H papers as many as needed. Add that household product directly onto the paper. Do not add the red cabbage juice when using the p. H paper! • Compare the color on the paper with the colors on the cover of the container. Record the p. H value of that household product. 8

• Dispose of the used p. H paper into the regular trash. • Dispose of the contents in the well-plates and the beaker in sink. • Wash all the glasswares, including the well-plates, beaker, medicine dropper, glass stirring rods, and watch glass, thoroughly with tap water. Return them to your bench for drying. • Wipe the bench with wet sponge…. . ask your instructor to sign you out…. then you are ready to leave. • Finish the lab report (including both data sheet and postlab questions) after the lab. Feel free to ask instructors questions, but never copy other students’ answers. • Turn in the lab report the next time when you are expected to come to the lab. • Don’t forget to prepare for the next lab before you come next time. 9
- Slides: 9