INTRO TO BIOCHEMISTRY THE ATOM THE PERIODIC TABLE

INTRO TO BIOCHEMISTRY : THE ATOM & THE PERIODIC TABLE Learning Goal: I will be able to recap what the structure of the atom looks like, how to draw Bohr-Rutherford diagrams, learn what isotopes and radioisotopes are, and how they are used in medicine, as well as how and why ions form, and be able to explain electronegativity

Elements � Elements on the Periodic Table are made up of identical atoms (the smallest whole particle that makes up matter) �e. g. A bar of the element gold contains trillions and trillions of identical gold atoms, all with identical structure

Atomic Structure � While the atoms of different elements are different, all atoms have the same basic structure: A dense positive nucleus of protons (+) and neutrons (0), surrounded by very small electrons ( -) on orbitals.
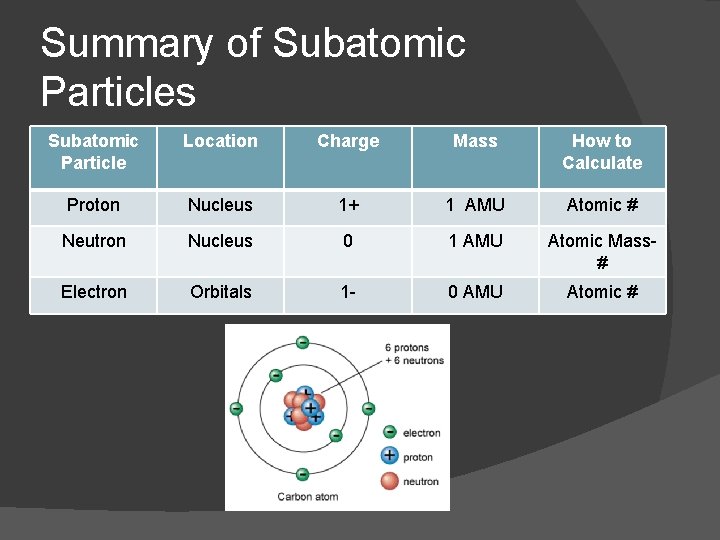
Summary of Subatomic Particles Subatomic Particle Location Charge Mass How to Calculate Proton Nucleus 1+ 1 AMU Atomic # Neutron Nucleus 0 1 AMU Atomic Mass# Electron Orbitals 1 - 0 AMU Atomic #
Bohr Rutherford Diagrams � Bohr Rutherford diagrams are used to show the arrangements of electrons (and the presence of protons and neutrons) in atoms � Steps for drawing Bohr-Rutherford diagrams: 1. Draw the nucleus. 2. Inside, write the # of protons (p) and # of neutrons (n). 3. Draw a larger circle around the nucleus to represent each energy level. 4. Show the electrons on the energy levels. (2, 8, 8)

Practice: Draw the Bohr Rutherford diagram for lithium How many valence (outer) Electrons does lithium have? How do you think this affects the reactivity of lithium?

You Try: Draw the Bohr Rutherford diagram for neon How many valence (outer) Electrons does neon have? How do you think this affects the reactivity of neon?
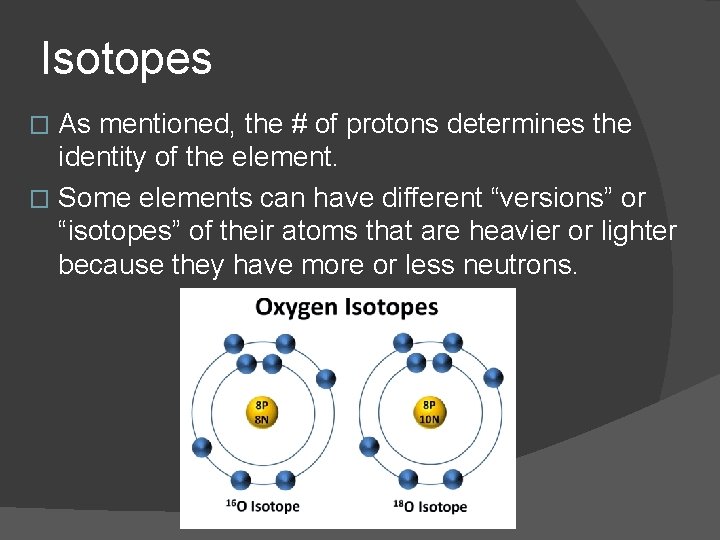
Isotopes As mentioned, the # of protons determines the identity of the element. � Some elements can have different “versions” or “isotopes” of their atoms that are heavier or lighter because they have more or less neutrons. �

Radioisotopes �Radioisotope ○ A version of a chemical element that has an unstable nucleus and emits radiation during its decay to a stable form. �Radioactivity ○ Radioactivity refers to the particles which are emitted from nuclei as a result of nuclear instability

Isotopes in Medicine � radioactive compounds are injected into the body so that images of cells can be scanned to diagnose and treat medical conditions such as cancer and heart disease. Radioisotopes may now be used so routinely and effectively that we have come to rely on them despite concerns about production safety https: //www. youtube. com/watch? v=E 4 B 94 z. CY 4 ok

ION FORMATION Atoms lose or gain electrons to try to have their outermost energy level completely full, or completely empty
Ion Formation � The number of protons and neutrons will stay the same. � Electrons will be lost or gained to establish a stable octet. �This means there will be a different number of protons vs electrons. �The atom will contain a charge �The charged atom is an ion.
Ion Formation � To achieve a stable octet, atoms can lose or gain electrons in 2 ways: �Transfer of electrons from one atom to another (ionic bonding) �Sharing electrons between two atoms (covalent bonding)

Ion Formation � The charge of an ion is determined by: �subtracting the number of electrons present in the atom from the number of protons. ○ A negative ion is an anion. ○ A positive ion is a cation.

…negatively charged atom a. Nion …positively charged atom ca ion
Ion Formation � It is easier for larger atoms to lose electrons because the electrons are held further away from the nucleus.

Example: Lithium’s Ion Formation ATOM ION 4 n 3 p Li Ionic Charge:
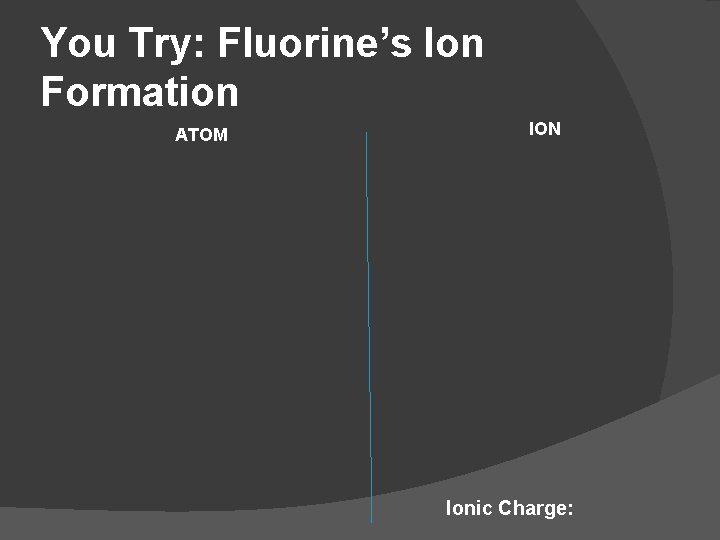
You Try: Fluorine’s Ion Formation ATOM ION Ionic Charge:

ATOMIC SIZE Atomic size refers to the size of the entire atom (not just the nucleus). this tells us how far away from the nucleus the electrons are located

Atomic Size � As you move down the groups in the periodic table, the atomic size increases. �as the number of layers of electrons increases, the outermost layer is further from the nucleus, so the atom gets bigger � As you move left-to-right across the periods, the atomic size decreases. �the increasing number of protons pulls the small electrons tighter to the nucleus, making the atom smaller
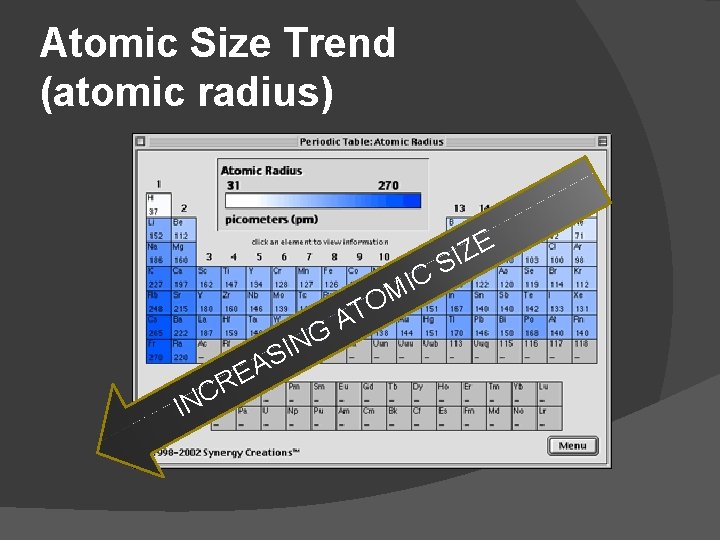
Atomic Size Trend (atomic radius) IN G N I S A E R C A IC M TO E Z I S

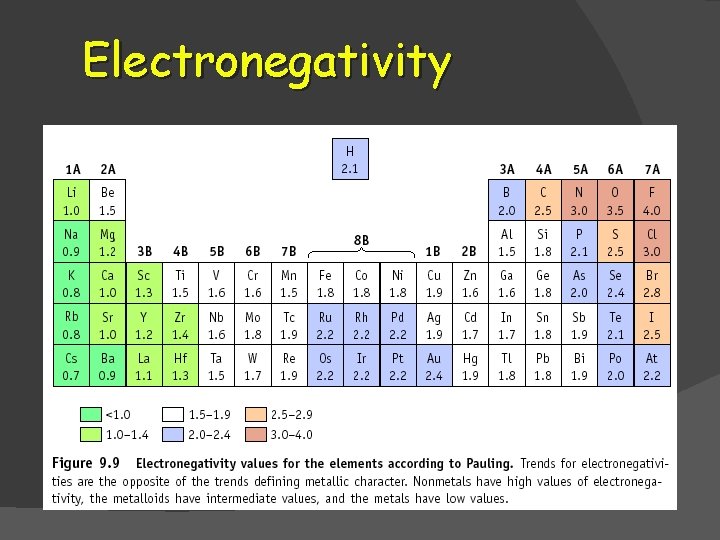
ELECTRONEGATIVITY The atom’s ability to attract electrons in a chemical bond. Each atom has an electronegativity value associated with it. The difference in electronegativity between two atoms in a bond determines the type of bond formed!
Electronegativity � As atomic size decreases, electronegativity increases. Why?

Electronegativity � The noble gases do not have a recorded electronegativity. �They do not bond with other atoms. �The outermost energy level contains a stable arrangement of electrons.
Electronegativity

Which is more electronegative? �F or Cl ? �Na or K ? �Sn or I ?

Electronegativity Differences in Molecules � EN = EN (first atom) – EN (second atom) EN is always a positive value.
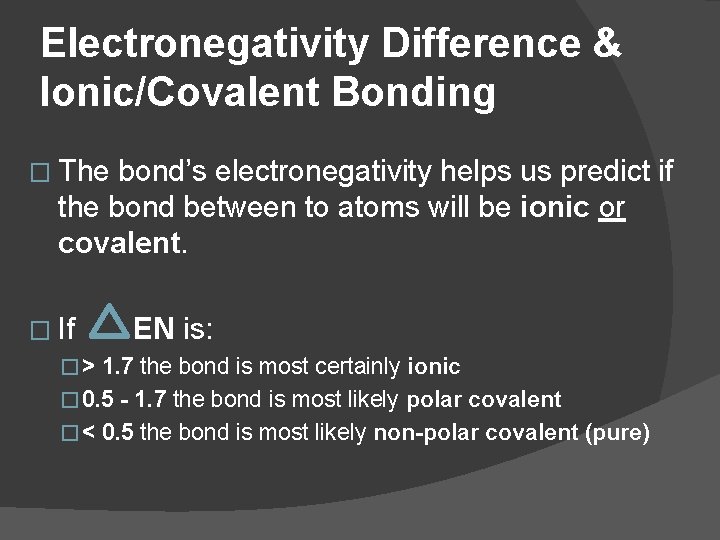
Electronegativity Difference & Ionic/Covalent Bonding � The bond’s electronegativity helps us predict if the bond between to atoms will be ionic or covalent. � If EN is: �> 1. 7 the bond is most certainly ionic � 0. 5 - 1. 7 the bond is most likely polar covalent �< 0. 5 the bond is most likely non-polar covalent (pure)

HOW DID WE DO? Learning Goal: I will be able to recap what the structure of the atom looks like, how to draw Bohr-Rutherford diagrams, learn what isotopes and radioisotopes are, and how they are used in medicine, as well as how and why ions form, and be able to explain electronegativity
- Slides: 29