INTRO AMI INTEGRILIN AND REDUCED DOSE OF THROMBOLYTIC
INTRO AMI INTEGRILIN AND REDUCED DOSE OF THROMBOLYTIC IN ACUTE MYOCARDIAL INFARCTION
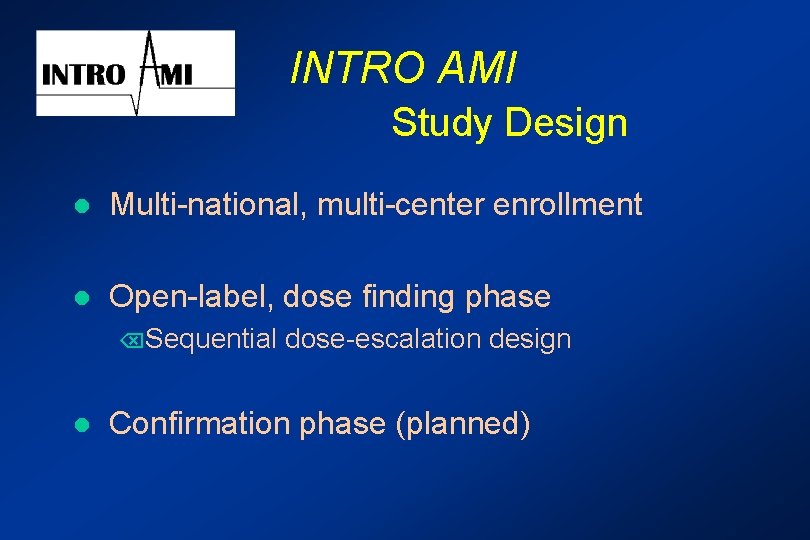
INTRO AMI Study Design l Multi-national, multi-center enrollment l Open-label, dose finding phase ÕSequential l dose-escalation design Confirmation phase (planned)
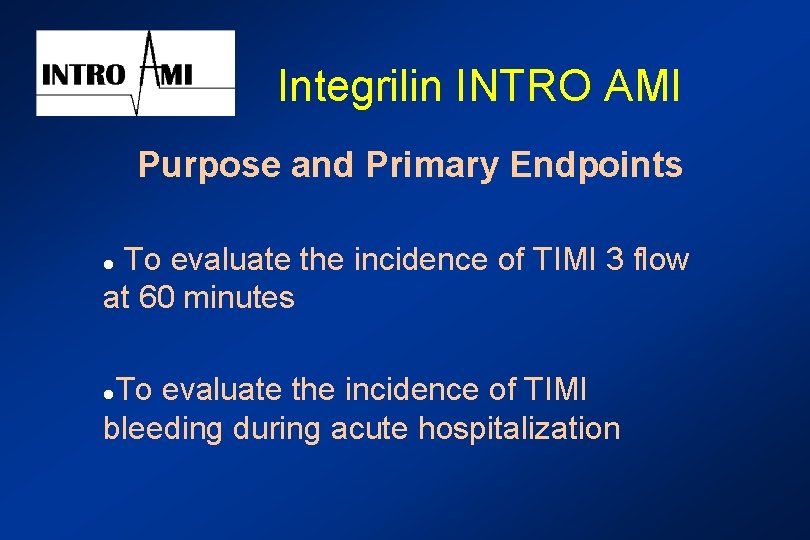
Integrilin INTRO AMI Purpose and Primary Endpoints To evaluate the incidence of TIMI 3 flow at 60 minutes l To evaluate the incidence of TIMI bleeding during acute hospitalization l
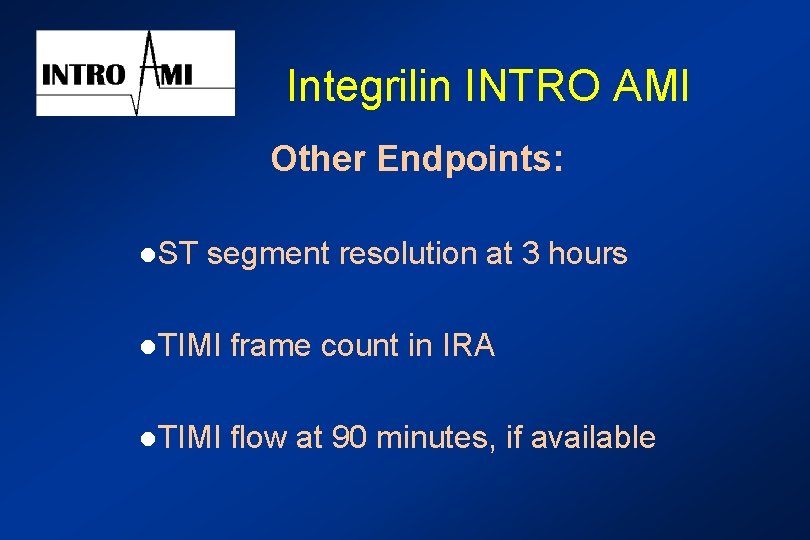
Integrilin INTRO AMI Other Endpoints: l. ST segment resolution at 3 hours l. TIMI frame count in IRA l. TIMI flow at 90 minutes, if available
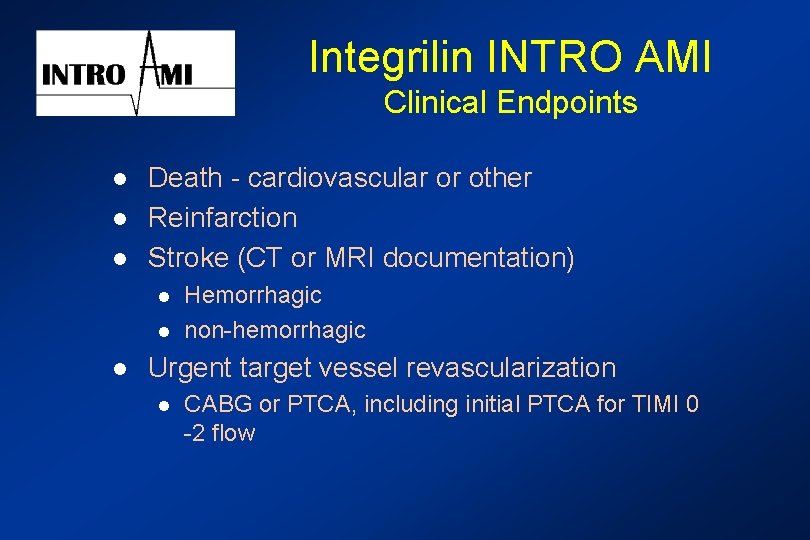
Integrilin INTRO AMI Clinical Endpoints l l l Death - cardiovascular or other Reinfarction Stroke (CT or MRI documentation) l l l Hemorrhagic non-hemorrhagic Urgent target vessel revascularization l CABG or PTCA, including initial PTCA for TIMI 0 -2 flow
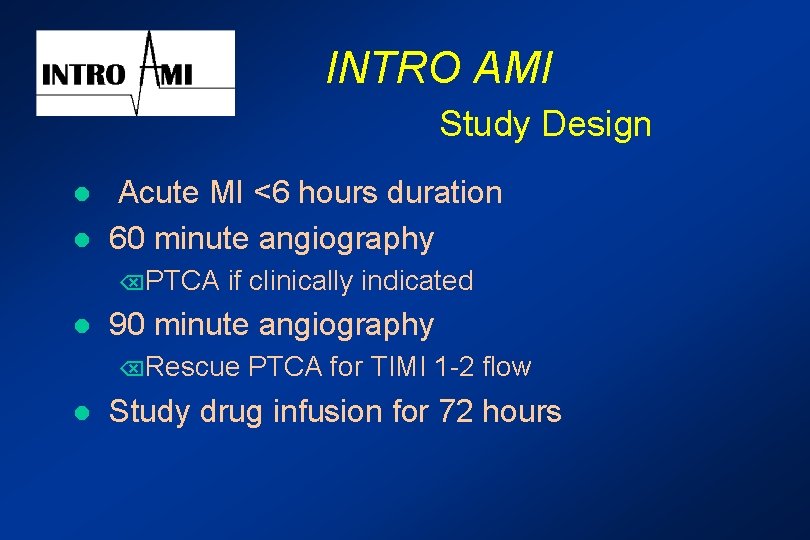
INTRO AMI Study Design l l Acute MI <6 hours duration 60 minute angiography ÕPTCA l if clinically indicated 90 minute angiography ÕRescue l PTCA for TIMI 1 -2 flow Study drug infusion for 72 hours

INTRO AMI Study Design l Heparin 4000 IU IV bolus Õ 800 IU/hr IV infusion Õa. PTT - 50 -70 seconds l ASA 325 mg / day l PCI at discretion of investigator

Integrilin Intro AMI Study Protocol
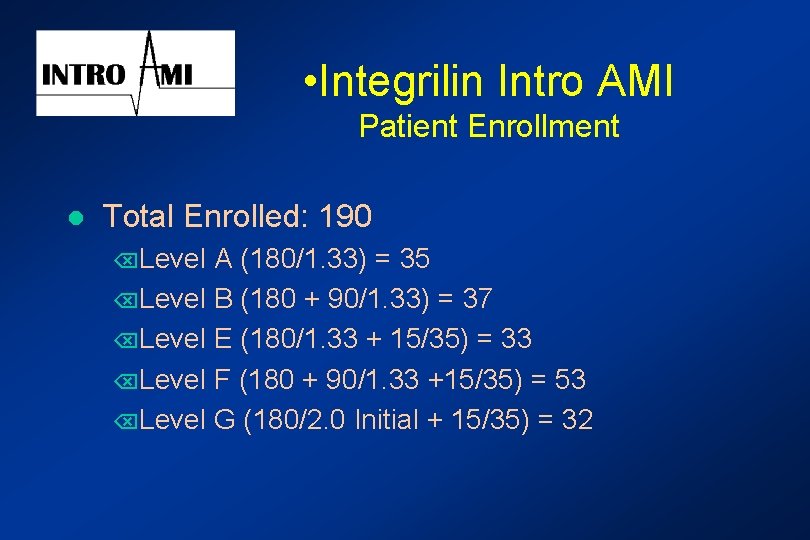
• Integrilin Intro AMI Patient Enrollment l Total Enrolled: 190 ÕLevel A (180/1. 33) = 35 ÕLevel B (180 + 90/1. 33) = 37 ÕLevel E (180/1. 33 + 15/35) = 33 ÕLevel F (180 + 90/1. 33 +15/35) = 53 ÕLevel G (180/2. 0 Initial + 15/35) = 32
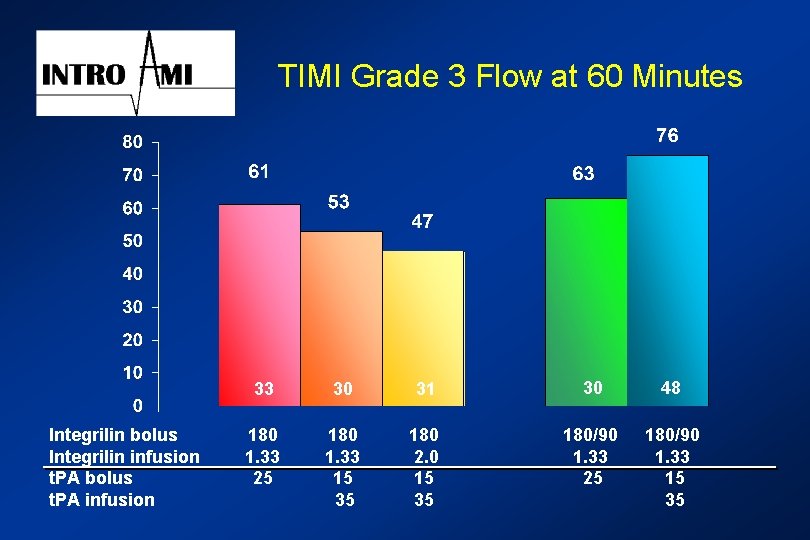
TIMI Grade 3 Flow at 60 Minutes Integrilin bolus Integrilin infusion t. PA bolus t. PA infusion 33 30 31 30 48 180 1. 33 25 180 1. 33 15 35 180 2. 0 15 35 180/90 1. 33 25 180/90 1. 33 15 35
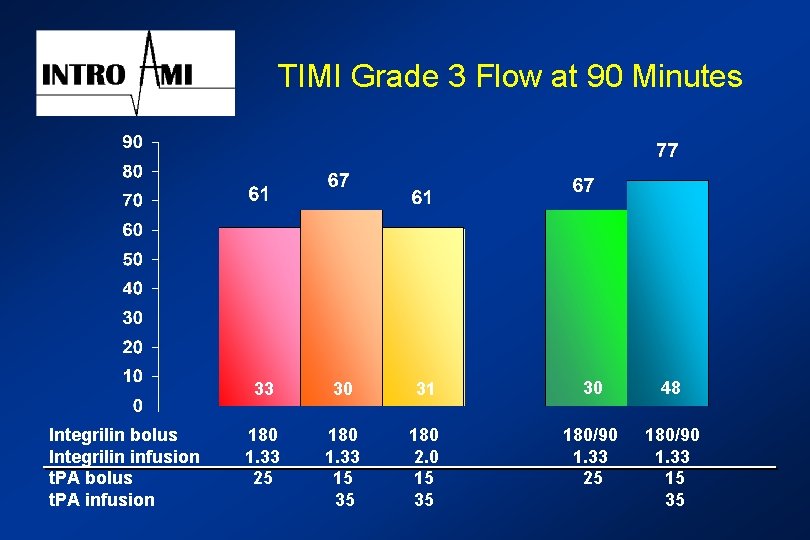
TIMI Grade 3 Flow at 90 Minutes Integrilin bolus Integrilin infusion t. PA bolus t. PA infusion 33 30 31 30 48 180 1. 33 25 180 1. 33 15 35 180 2. 0 15 35 180/90 1. 33 25 180/90 1. 33 15 35

Bleeding events Site adjudication l Level A (N=35) l Õ Moderate - 2 pts. Õ Severe - 3 pts. l Level E (N = 33) Õ Moderate -5 Õ Severe - 2 l Level G (N = 32) Õ Moderate - 1 pts. Õ Severe - 0 pts Level B (N=35) Õ Moderate - 6 pts. Õ Severe - 2 pts. l Level F (N = 53) Õ Moderate -5 Õ Severe - 0

• Preliminary Results - OVERALL TIMI Major Bleeding Events l Single bolus Integrilin Õ Level l Double bolus Integrilin Õ Level A = 3/35 • CABG related = 3 • CABG related = 1 Õ Level E = 3/33 F = 7/53 • CABG related = 4 • CABG related = 1 Õ Level B = 6/35 G = 6/32 • CABG related = 2 l 12/100 Õ 4 CABG related l 13/88 Õ 7 CABG related
Integrilin INTRO AMI Summary and Conclusions l Efficacy l l l The combination of Integrilin and reduced dose rt-PA achieves enhanced TIMI 3 flow at 60 and 90 minutes. Greatest increase in TIMI 3 flow results from a double bolus of Integrilin and with half-dose rt. PA (15/35 mg). Safety l There appears to be a clinically acceptable increase in bleeding related primarily to femoral access site.

Integrilin INTRO AMI Conclusions l The use of Integrilin in combination with low dose fibrinolytics is a promising therapeutic strategy in acute myocardial infarction. l Large scale clinical-outcome studies will be required to further assess the benefits of this strategy.
- Slides: 15