Intrepid Transcatheter Mitral Valve Replacement Design Clinical Results








- Slides: 19

Intrepid Transcatheter Mitral Valve Replacement Design, Clinical Results and Next Steps Michael J Mack, MD, FACC The Heart Hospital Baylor Plano Dallas, TX

Conflict of Interest Disclosure • Abbott Vascular- Co PI COAPT Trial • Medtronic- Study Chair-APOLLO Trial • Edwards Lifesciences- Co-PI PARTNER 3 Trial CRT. 18

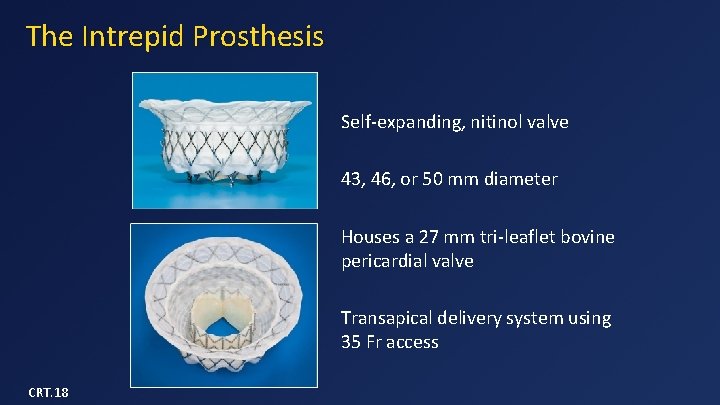
The Intrepid Prosthesis Self-expanding, nitinol valve 43, 46, or 50 mm diameter Houses a 27 mm tri-leaflet bovine pericardial valve Transapical delivery system using 35 Fr access CRT. 18

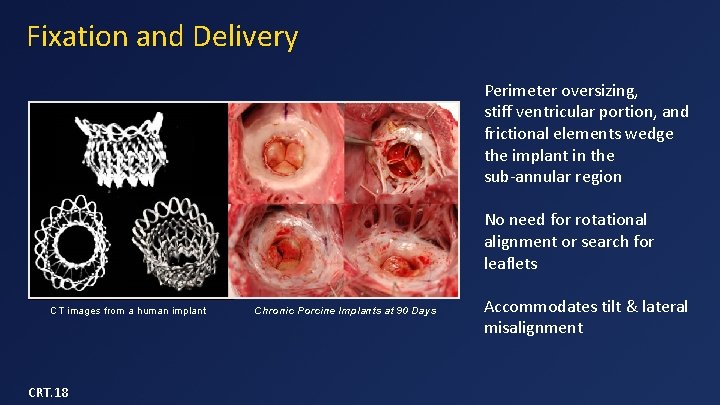
Fixation and Delivery Perimeter oversizing, stiff ventricular portion, and frictional elements wedge the implant in the sub-annular region No need for rotational alignment or search for leaflets CT images from a human implant CRT. 18 Chronic Porcine Implants at 90 Days Accommodates tilt & lateral misalignment

Pilot Study Design Study Aim • To determine the feasibility of TMVR with the Intrepid valve Analysis Cohort • The initial 50 consecutively enrolled patients in the pilot study (06 May 2015 to 21 July 2017) Clinical Endpoints • MVARC criteria • An independent physician committee reviewed adverse clinical events, including mortality, stroke, myocardial infarction, bleeding, re-hospitalization, and reoperation CRT. 18

Participating Sites Abbott NW Minneapolis, MN Aurora St. Luke's Milwaukee, WI U of Michigan Ann Arbor, MI St. Thomas’ Hospital NYU Langone London, UK New York, NY Helsinki University Hospital Helsinki, Finland Leeds Teaching Hospitals Leeds, UK Mount Sinai John Paul II Hospital* New York, NY Columbia University New York, NY Krakow, Poland Brighton and Sussex University Hospitals Brighton, UK Barnes Jewish St. Louis, MO Piedmont Atlanta, GA Baylor Heart and Vascular Centre Hospitalier Regional Univeritaire de Lille, France Clinique Pasteur Dallas, TX Toulouse, France Northwestern University Chicago, Il Royal Prince Alfred Hospital Houston Methodist Sydney, Australia Houston, TX The Alfred Melbourne, Australia CRT. 18 *First in human Monash Heart Melbourne, Australia Hygeia Hospital Athens, Greece

JACC Volume 71, Issue 1, January 2018 Intrepid Global Pilot Study

Inclusion and Exclusion Criteria Key Inclusion Criteria • • • Symptomatic, severe mitral regurgitation Deemed high or extreme surgical risk by the local heart team Native mitral valve geometry and size compatible with the Intrepid™ TMVR Mild or no mitral valve calcification LVEF ≥ 20% Key Exclusion Criteria • • • CRT. 18 Pulmonary hypertension (systolic pressure ≥ 70 mm Hg) Need for coronary revascularization Hemodynamic instability Need for other valvular therapy Severe renal insufficiency (Cr > 2. 5 mg/dl) Prior MV surgery or intervention

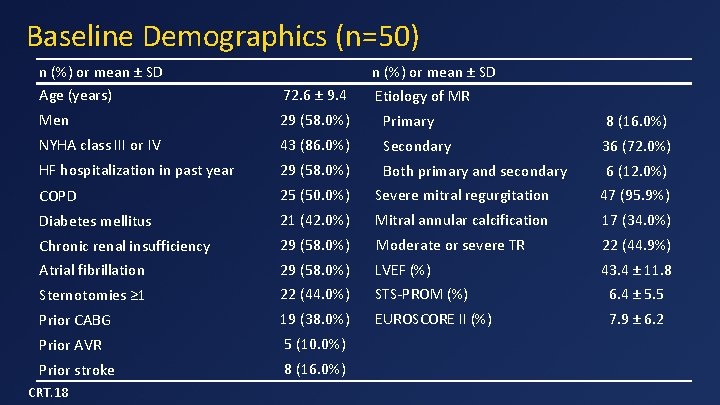
Baseline Demographics (n=50) n (%) or mean ± SD Age (years) 72. 6 ± 9. 4 Men 29 (58. 0%) Primary 8 (16. 0%) NYHA class III or IV 43 (86. 0%) Secondary 36 (72. 0%) HF hospitalization in past year 29 (58. 0%) Both primary and secondary 6 (12. 0%) COPD 25 (50. 0%) Severe mitral regurgitation 47 (95. 9%) Diabetes mellitus 21 (42. 0%) Mitral annular calcification 17 (34. 0%) Chronic renal insufficiency 29 (58. 0%) Moderate or severe TR 22 (44. 9%) Atrial fibrillation 29 (58. 0%) LVEF (%) 43. 4 ± 11. 8 Sternotomies ≥ 1 22 (44. 0%) STS-PROM (%) 6. 4 ± 5. 5 Prior CABG 19 (38. 0%) EUROSCORE II (%) 7. 9 ± 6. 2 Prior AVR 5 (10. 0%) Prior stroke 8 (16. 0%) CRT. 18 n (%) or mean ± SD Etiology of MR

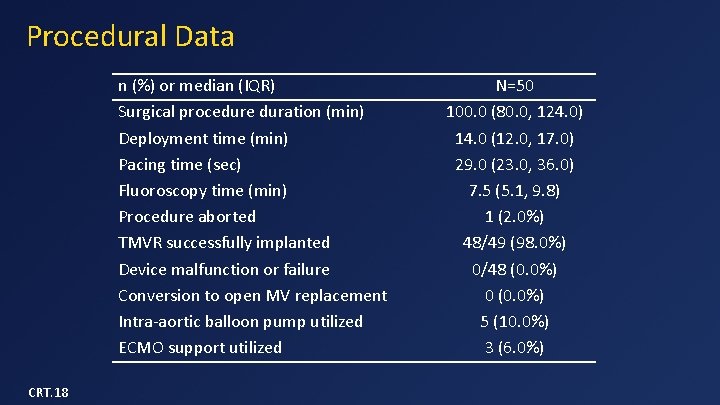
Procedural Data n (%) or median (IQR) Surgical procedure duration (min) Deployment time (min) Pacing time (sec) Fluoroscopy time (min) Procedure aborted TMVR successfully implanted Device malfunction or failure Conversion to open MV replacement Intra-aortic balloon pump utilized ECMO support utilized CRT. 18 N=50 100. 0 (80. 0, 124. 0) 14. 0 (12. 0, 17. 0) 29. 0 (23. 0, 36. 0) 7. 5 (5. 1, 9. 8) 1 (2. 0%) 48/49 (98. 0%) 0/48 (0. 0%) 0 (0. 0%) 5 (10. 0%) 3 (6. 0%)

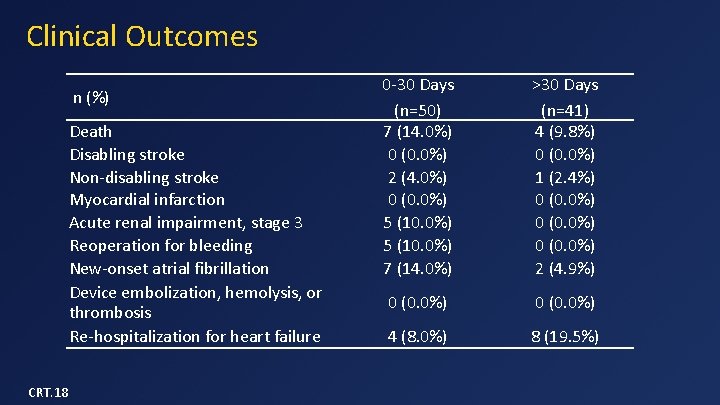
Clinical Outcomes n (%) Death Disabling stroke Non-disabling stroke Myocardial infarction Acute renal impairment, stage 3 Reoperation for bleeding New-onset atrial fibrillation Device embolization, hemolysis, or thrombosis Re-hospitalization for heart failure CRT. 18 0 -30 Days (n=50) 7 (14. 0%) 0 (0. 0%) 2 (4. 0%) 0 (0. 0%) 5 (10. 0%) 7 (14. 0%) >30 Days (n=41) 4 (9. 8%) 0 (0. 0%) 1 (2. 4%) 0 (0. 0%) 2 (4. 9%) 0 (0. 0%) 4 (8. 0%) 8 (19. 5%)

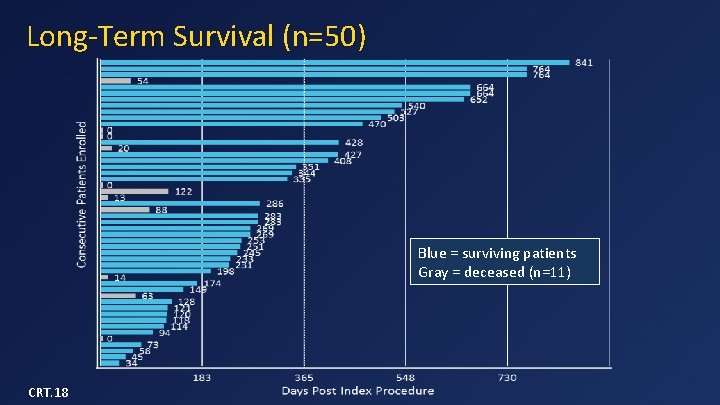
Long-Term Survival (n=50) Blue = surviving patients Gray = deceased (n=11) CRT. 18

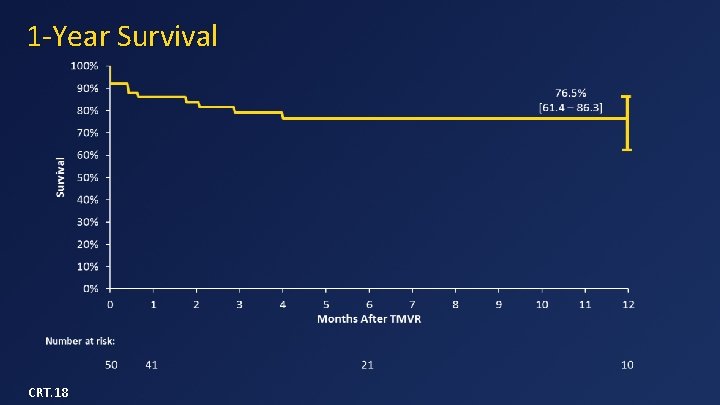
1 -Year Survival CRT. 18

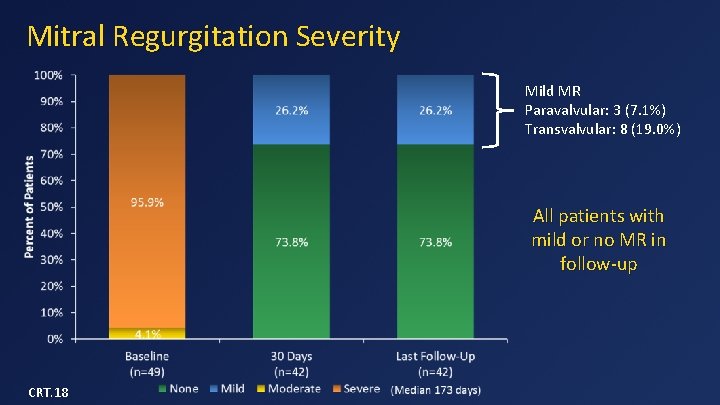
Mitral Regurgitation Severity Mild MR Paravalvular: 3 (7. 1%) Transvalvular: 8 (19. 0%) All patients with mild or no MR in follow-up CRT. 18

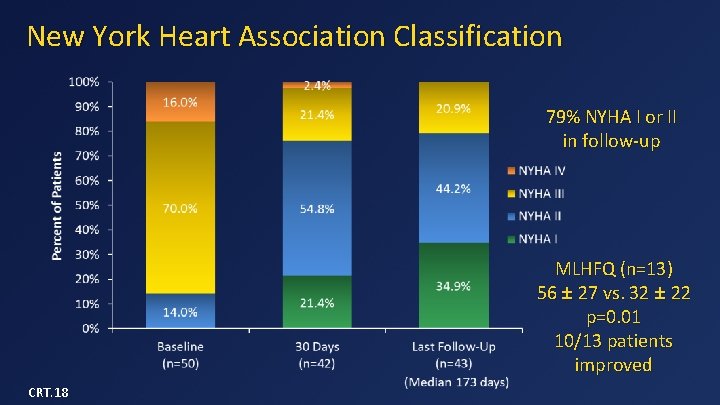
New York Heart Association Classification 79% NYHA I or II in follow-up MLHFQ (n=13) 56 ± 27 vs. 32 ± 22 p=0. 01 10/13 patients improved CRT. 18

Data Summary (n=50) • Device implant success in 48/49 (98%) • 30 -day mortality = 14% – 3 from apical bleeding, 3 from CHF, 1 from malposition • One-year survival = 77% – 3 SCDs in patients with low EF and no ICDs – No death after 180 days • No device malfunction, hemolysis, or thrombosis • No or mild MR in all survivors • 79% of patients in NYHA class I or II in follow-up CRT. 18

Pilot Study Summary • TMVR with the Intrepid valve was feasible and resulted in correction of MR in symptomatic patients at high or extreme surgical risk • Stable valve function was observed, and a majority of patients experienced symptom improvement • Further investigations will determine the role of this therapy in a broader patient population, compared with surgery and other transcatheter techniques CRT. 18

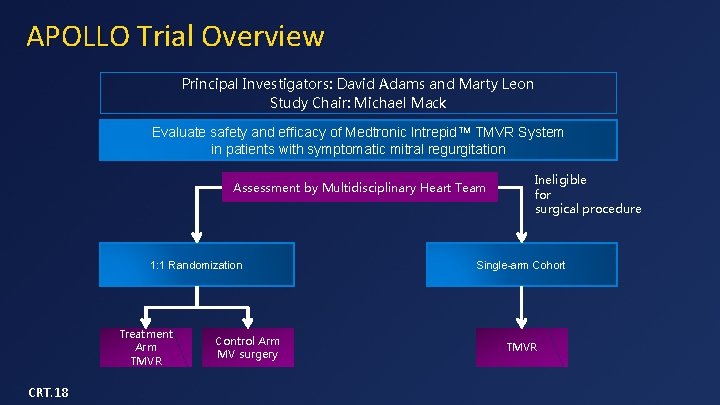
APOLLO Trial Overview Principal Investigators: David Adams and Marty Leon Study Chair: Michael Mack Evaluate safety and efficacy of Medtronic Intrepid. TM TMVR System in patients with symptomatic mitral regurgitation Assessment by Multidisciplinary Heart Team 1: 1 Randomization Treatment Arm TMVR CRT. 18 Control Arm MV surgery Ineligible for surgical procedure Single-arm Cohort TMVR

Highlights • • First patient implant in the APOLLO Trial was October 2017 All sites are projected for activation by Spring 2018 Site screening activities are ramping with activation and initiations Transfemoral is anticipated for initial human use in 2018 CRT. 18