INTREPID Status of the Apollo Trial Clinical Results


- Slides: 15

INTREPID: Status of the Apollo Trial, Clinical Results and Next Steps Michael J. Reardon, M. D. Professor of Cardiothoracic Surgery Allison Family Distinguished Chair of Cardiovascular Research Houston Methodist Hospital

Michael J. Reardon, M. D. Advisory Board/ consultant Medtronic, Boston Scientific, Gore Medical

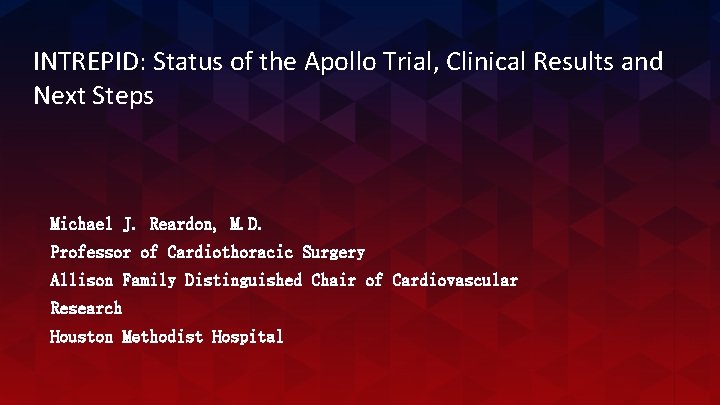
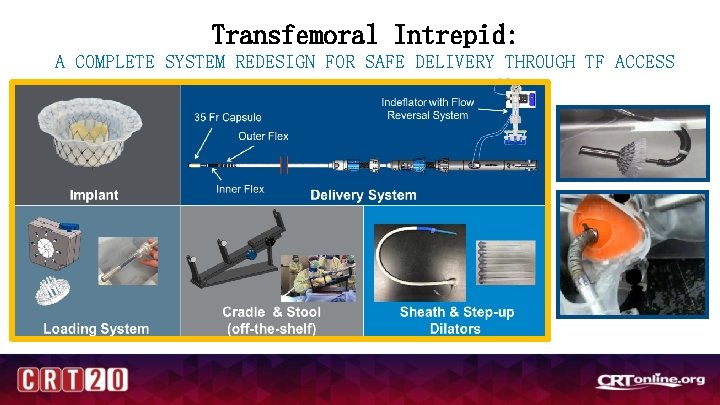
The Intrepid Valve Conformable and symmetric design eliminates the need for rotational orientation and simplifies device implantation Flexible atrial brim facilitates visualization during implant & subsequent tissue in-growth Dual-stent design engages the dynamic valve anatomy while allowing the inner frame maintain circularity independent of cardiac cycle or annulus shape

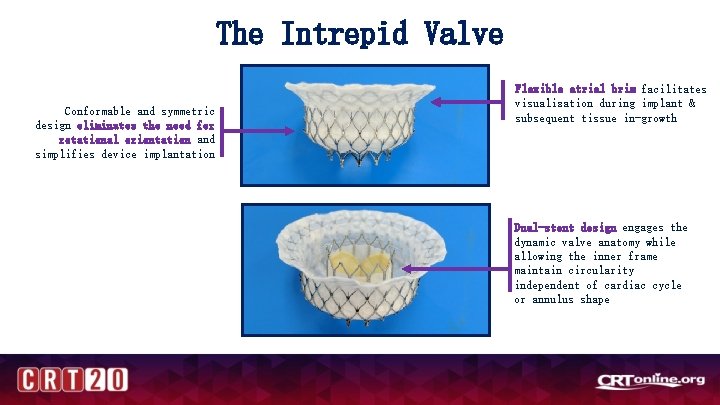
The APOLLO Trial PIs: Martin B. Leon, CUMC (NYC) & David Adams, MSMC (NYC) Study Chair: Michael Mack, Baylor (Dallas) Evaluate safety and efficacy of Medtronic Intrepid. TM TMVR System in patients with severe symptomatic mitral regurgitation (1 ry or 2 ry MR) Assessment by Multidisciplinary Heart Team Ineligible for surgery Eligible for surgery Roll-in subjects N=120 -180 1: 1 Randomization N=300, 400, 500 (stratified Primary and Secondary MR) Treatment Arm TMVR Primary Endpoint: Control Arm MV surgery Single-arm Cohort N=200, 300, 400, 500 (Primary and Secondary MR) TMVR Analysis Cohort TMVR MAC Cohort All-cause mortality, disabling stroke, reoperation (or reintervention), and cardiovascular hospitalization at 1 year

The APOLLO TRIAL ENROLLMENT STATUS 65+ 800+ 200+ SITES ACTIVATED PATIENTS CONSENTED PATIENTS APPROVED LARGE & GROWING GLOBAL CLINICAL EXPERIENCE PATIENT SELECTION VIA RIGOROUS SCREENING & CASE REVIEW METICULOUS PRE & POST OPERATIVE CARE INITIATIVES

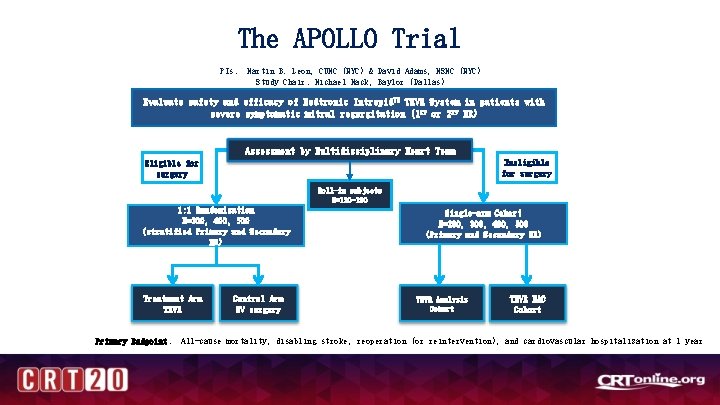
The APOLLO Trial PIs: Martin B. Leon, CUMC (NYC) & David Adams, MSMC (NYC) Study Chair: Michael Mack, Baylor (Dallas) INTREDPID IN MAC Evaluate safety and efficacy of Medtronic Intrepid. TM TMVR System in patients with severe symptomatic mitral regurgitation (1 ry or 2 ry MR) • Assessment by Multidisciplinary Heart Team Ineligible for surgery Eligible for surgery Roll-in subjects N=120 -180 1: 1 Randomization N=300, 400, 500 (stratified 1 ry and 2 ry MR) Single-arm Cohort N=200, 300, 400, 500 (1 ry and 2 ry MR) • • • Control Arm MV surgery TMVR Analysis Cohort TMVR MAC Registry Severe MR + MAC Moderate MR with MS + MAC 1 -year composite endpoint: • • • Treatment Arm TMVR Approved MAC cohort for patients with: All-cause mortality Heart failure hospitalization Up to 300 patients

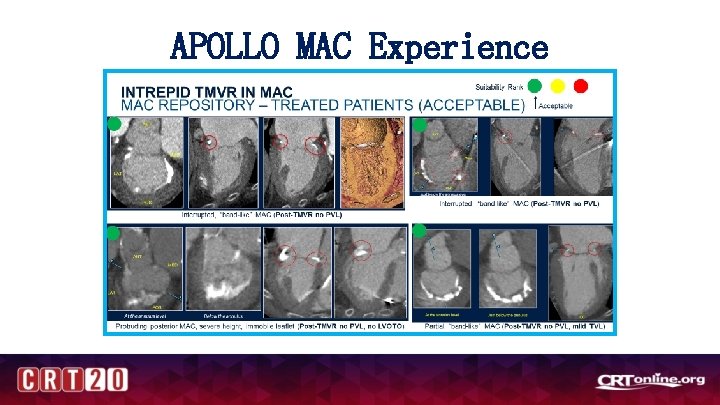
APOLLO MAC Experience

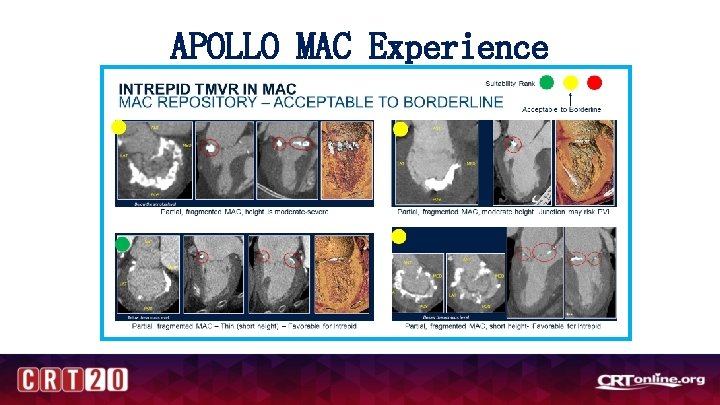
APOLLO MAC Experience

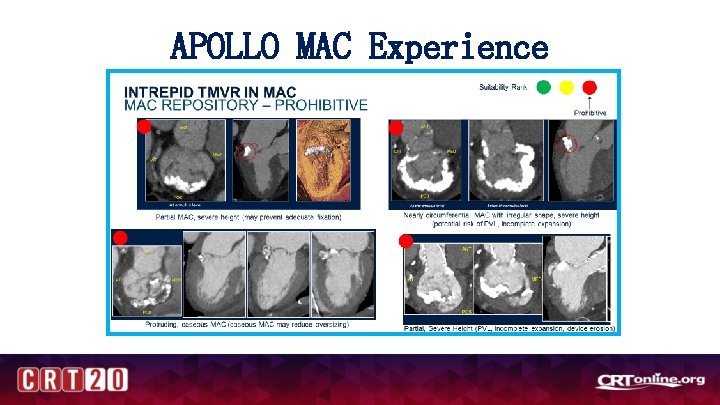
APOLLO MAC Experience

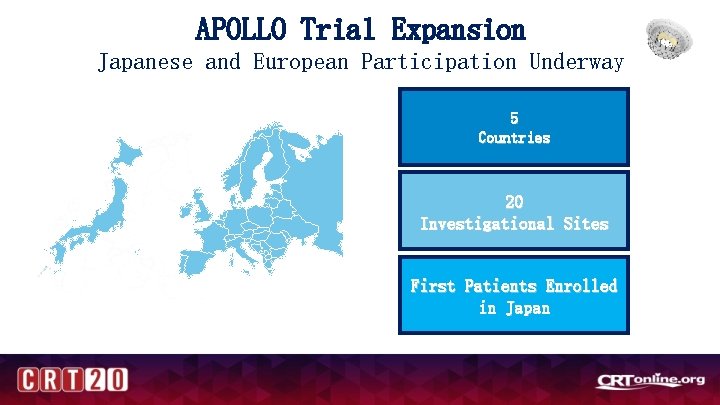
APOLLO Trial Expansion Japanese and European Participation Underway 5 Countries 20 Investigational Sites First Patients Enrolled in Japan

Transfemoral Intrepid: A COMPLETE SYSTEM REDESIGN FOR SAFE DELIVERY THROUGH TF ACCESS

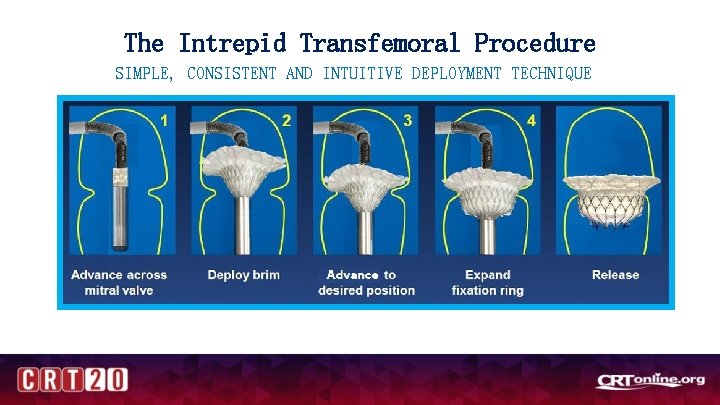
The Intrepid Transfemoral Procedure SIMPLE, CONSISTENT AND INTUITIVE DEPLOYMENT TECHNIQUE

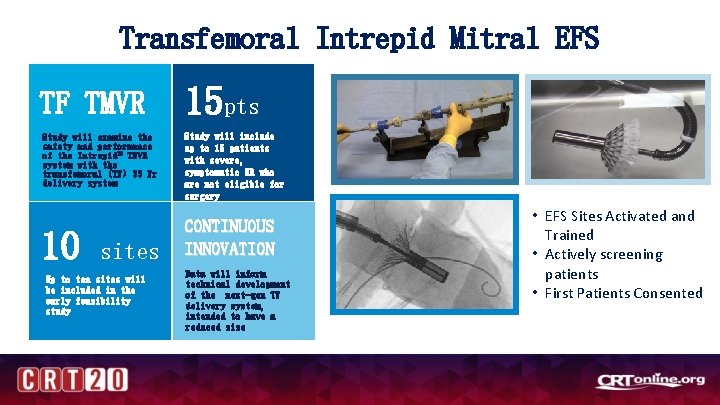
Transfemoral Intrepid Mitral EFS TF TMVR Study will examine the safety and performance of the Intrepid™ TMVR system with the transfemoral (TF) 35 Fr delivery system 10 sites Up to ten sites will be included in the early feasibility study 15 pts Study will include up to 15 patients with severe, symptomatic MR who are not eligible for surgery CONTINUOUS INNOVATION Data will inform technical development of the next-gen TF delivery system, intended to have a reduced size • EFS Sites Activated and Trained • Actively screening patients • First Patients Consented

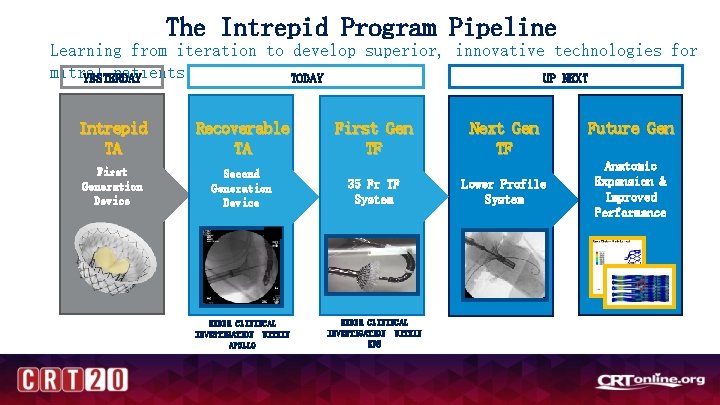
The Intrepid Program Pipeline Learning from iteration to develop superior, innovative technologies for mitral patients YESTERDAY TODAY UP NEXT Intrepid TA Recoverable TA First Generation Device Second Generation Device 35 Fr TF System UNDER CLININCAL INVESTIGATION WITHIN APOLLO UNDER CLININCAL INVESTIGATION WITHIN EFS APOLLO First Gen TF EFS Approved Next Gen TF Lower Profile System Future Gen Anatomic Expansion & Improved Performance

Thank You