INTRAVENOUS ADNUXTURE 1 Introduction Intravenous admixture is the









- Slides: 32

INTRAVENOUS ADNUXTURE 1

Introduction • Intravenous admixture is the combination of two or more parenteral products in one container • Why. . – The combination of drug substances in an IV. fluid can promote parenteral incompatibilities. – Hospital pharmacists can develop the expertise to prepare these solutions; recognizing their compatibility, stability problems and the potential for contamination and participate in administration of the solutions. – The complex compounding requires knowledgeable personnel making accurate calculations, compounding and having aseptic technique. 2

• IV admixture systems: – intravenous fluids, – intravenous administration systems, – administration methods, – flow rate of intravenous fluids, – techniques for preparation of admixtures and – admixture programs. 3

Intravenous Fluids I- Large-volume intravenous solutions: • The injections intended for IV use, are packaged in containers holding 100 ml or more. • Other sterile large volume solutions include – Solution used for irrigation or for dialysis. These may be packaged in containers designed to empty rapidly and may contain a volume of more than 100 ml. • They are packed in single-dose units in suitable glass or plastic containers. • In addition to being sterile, they are pyrogen-free and free of particulate matter. • Bacteriostatic agents are never included, in order to avoid the toxicity due to the large volume administered. 4

Intravenous Fluids I- Large-volume intravenous solutions: • Although it is desirable that IV fluid be isotonic, hypertonic solutions can be administered successfully. • Highly concentrated hypertonic nutrient solutions are being used in parenteral hyperalimentation. Continuous IV infusions should also be included in the I. V. admixture program. Several continuous I. V. solutions are available commercially: – – glucose 5 D/w; normal solution saline; ringer's, lactated ringer's, sodium bicarbonate, ammonium chloride, sodium lactate, levulose, invert sugar; mannitol, alcohol, – dextran 40, dextran 70 and multiple electrolyte so 1 utions. 5

2 - Additives: The additives are injections packaged in ampoules or vials, prefilled or sterile solids, the latter are reconstituted with a suitable diluent before addition to the I. V. fluid. A fresh, sterile, disposable syringe is used for each additive. Ampoules: – – – 6 Not required to contain an antimicrobial preservative. In contrast to vials, ampoules-do -not provide for dosage flexibility. Ampoules are opened by- scoring the neck at the point of constriction with an ampoule file in order to break off the top. For larger ampoules, the opening has been made easier by prescoring or inscribing the ampoule neck with a circle of ceramic point.

2 - Additives: disadvantage of the glass ampoule – the contamination of the injection by glass particles when the container is opened. – the ampoule is inconvenient to the user because the content must be transferred to a syringe prior to administration Vials: The availability of multiple-dose vials sealed with rubber closure permitted flexibility of dosage and reduced the unit cost per dose. Disadvantages – 7 increased possibility of microbial contamination with repeated withdrawal and increased particulate contamination.

Sterile solid: drugs supplied by the manufacturer in solid form must be reconstituted before use. Prefilled syringes: Some injectable products are packaged in pre-filled syringes with or without special administration devices. Each prefilled unit contains a dose of medication with an attached sterile needle. After administration, the cartridge-needle unit is discarded, the injection is reusable. 8

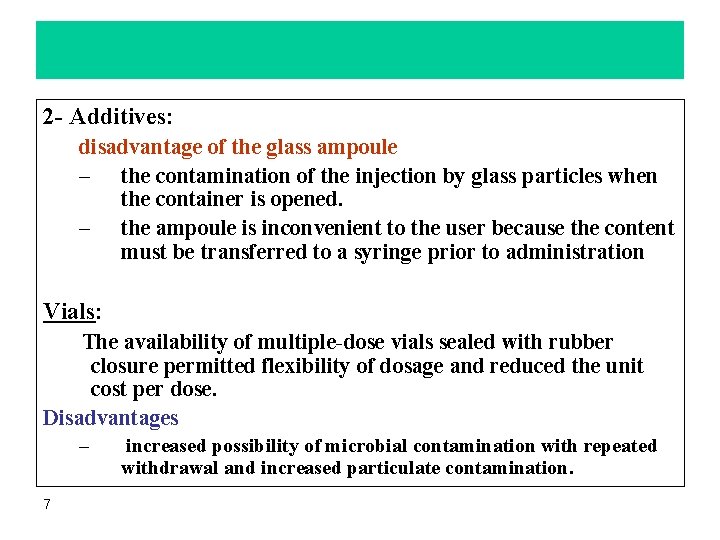
Intravenous administration systems IV fluids are available in glass and plastic containers. They are single-dose and should be discarded after opening even if not used. The plastic containers made from either a flexible or semirigid plastic material and nonvented. – – 9 Plastic is less expensive and is less dangerous if a hanging bottle fall. the plastic containers should be translucent and require very careful inspection concerning solution-container interactions, stability, freedom from particulate matter. Plastic containers have-eyelet openings or plastic-straps for attachment to IV- poles. flexible plastic systems don't require air introduction in order to function. Atmospheric pressure pressing on the container forces the fluid to flow.

Intravenous administration systems 10

IV fluid systems utilizing glass bottles – – – operate by gravity flow with room air replacing the lost volume of fluid administered. The bottles are graduated at 20 -ml increments scales that permit the volume in container determined either from upright or inverted position. Glass containers have aluminum and plastic bands for hanging. Fluids for IV are available from 3 sources viz Baxter, Mc. Gaw and Abbott/Cutter. – – 11 The major difference between the various manufactures of IV fluid systems utilizing glass bottles is the presence of an airway in the bottle. As mentioned, all IV fluids flow by gravity and the fluid empties from the bottle replaced by air from surrounding atmosphere.

Baxter and Mc. Gaw systems • utilize a plastic airway tube that extends from the rubber stopper above the level of the fluid when the bottle is hanging for administration. • Due to the airway the air that comes into the bottle does not pass through the fluid and this reduces the danger of oxidation the drugs in the fluid. disadvantage of this system • the airway does not contain any mechanisms for filtering. • Both companies counter this idea with the fact that any contaminants which might entrance to the bottle will lay on the top of the solution and remain there throughout the period of fluid therapy since there is some amount of fluid in the bottle and the administration set that is not administered, these contaminants will remain in the bottle and not administered to the patient. The Abbott /Cutter systems • both utilize a filtered airway on the administration set for the admission of air to the bottle. This filter has the capability of filtering some microorganisms from the air before the air the fluid. 12

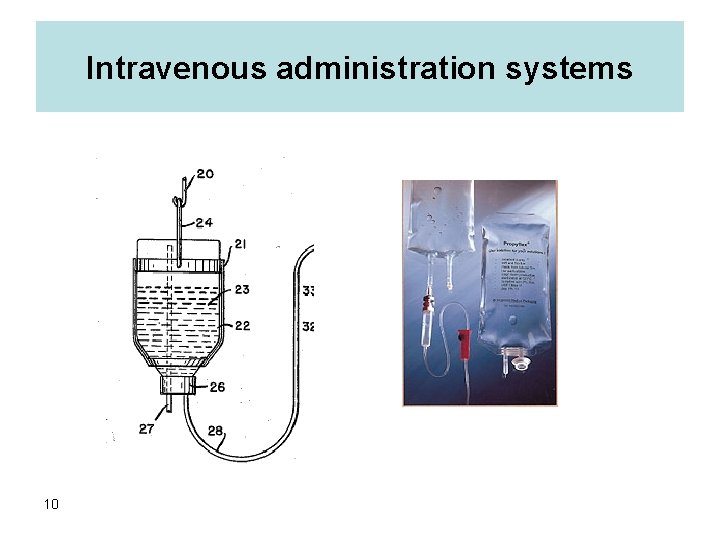
Each administration set (Baxter and Mc. Gaw or Abbott / Cutter) consists of several parts: – an airway for admitting air into the bottle (in the Baxter/Mc Gaw systems this is not an-integral part of set), – a drip chamber for counting the drops to determine the flow rate and also to act as a reservoir to insure continuous flow, – a length of tubing of sufficient length to reach from hanging bottle to patient arm, – a flow control meter – an injection site for adding medications to the bottle of fluid – a site for the attachment of the needle or other auxiliary equipment, e. g. , final filters, small scallp vein sets, to the administration set. These various parts are presented in the following diagram 13

14

• The device most frequently used for penetrating the skin is a hallow needle made from stainless steel. • Needle sizes are specified by the length of cannula and guage. Needles range in length from 3/8 to 6 inch. The gauge of the needle referes to the outside diameter (O. D. ) of the shaft. • Hollow needles are available in sizes from 13 - to 27 gauge. – 13 gauge needle has O. D. of 0. 095 inch, whereas – 27 gauge needle has O-D. of 0. 016 inch, • thus the larger the gauge of the needle, the smaller the diameter of lumen. 15

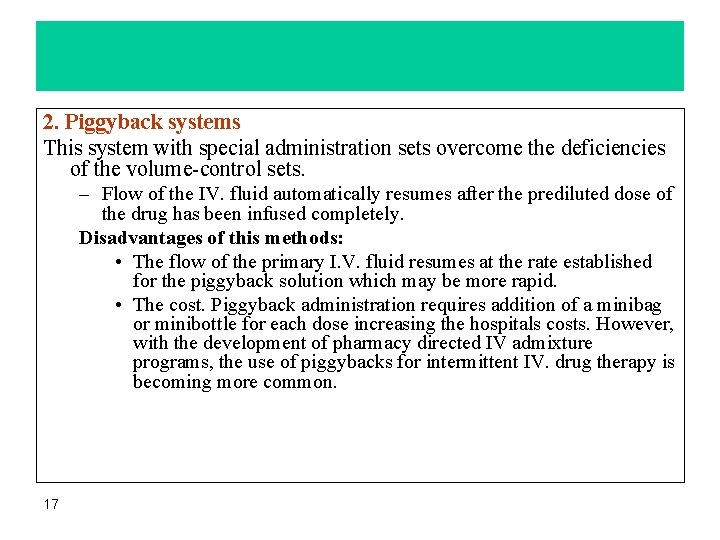
Administration methods Both Volume-control sets or piggyback methods 1. Volume-control systems • Allow IV. fluids to be diluted further and administered at a slower rate. • have a cost advantage compared with piggyback systems. • require manual readjustment to restart the flow of the main IV. solution when the medication in the chamber has been completely infused. • Disadvantages of volume-control sets – catheter occlusion- or drug dilution may result, if this function is not performed in a timely manner. – Other problems associated with volume-control sets are drug incompatibility and instability, and assuring proper dilution 16

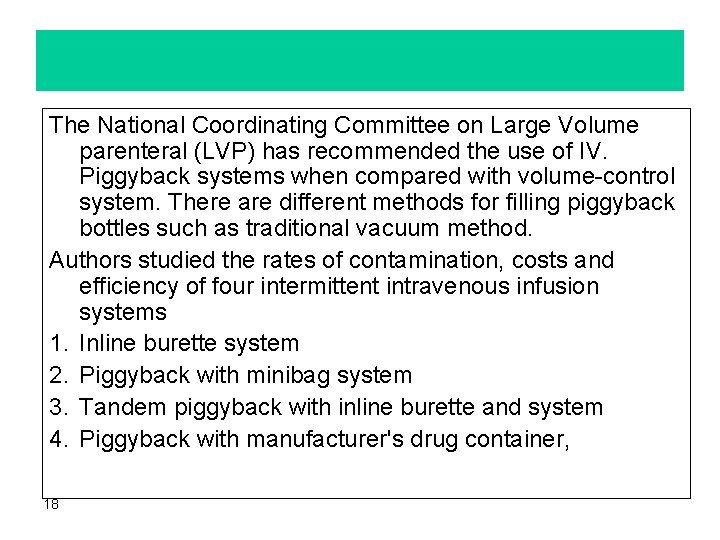
2. Piggyback systems This system with special administration sets overcome the deficiencies of the volume-control sets. – Flow of the IV. fluid automatically resumes after the prediluted dose of the drug has been infused completely. Disadvantages of this methods: • The flow of the primary I. V. fluid resumes at the rate established for the piggyback solution which may be more rapid. • The cost. Piggyback administration requires addition of a minibag or minibottle for each dose increasing the hospitals costs. However, with the development of pharmacy directed IV admixture programs, the use of piggybacks for intermittent IV. drug therapy is becoming more common. 17

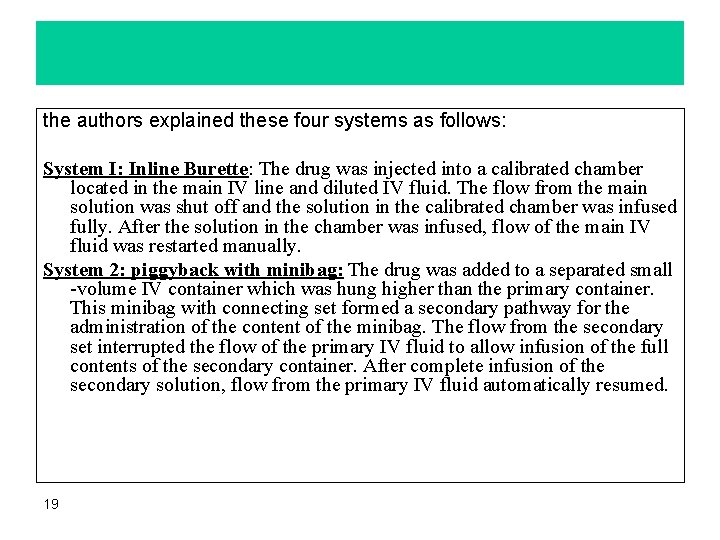
The National Coordinating Committee on Large Volume parenteral (LVP) has recommended the use of IV. Piggyback systems when compared with volume-control system. There are different methods for filling piggyback bottles such as traditional vacuum method. Authors studied the rates of contamination, costs and efficiency of four intermittent intravenous infusion systems 1. Inline burette system 2. Piggyback with minibag system 3. Tandem piggyback with inline burette and system 4. Piggyback with manufacturer's drug container, 18

the authors explained these four systems as follows: System I: Inline Burette: The drug was injected into a calibrated chamber located in the main IV line and diluted IV fluid. The flow from the main solution was shut off and the solution in the calibrated chamber was infused fully. After the solution in the chamber was infused, flow of the main IV fluid was restarted manually. System 2: piggyback with minibag: The drug was added to a separated small -volume IV container which was hung higher than the primary container. This minibag with connecting set formed a secondary pathway for the administration of the content of the minibag. The flow from the secondary set interrupted the flow of the primary IV fluid to allow infusion of the full contents of the secondary container. After complete infusion of the secondary solution, flow from the primary IV fluid automatically resumed. 19

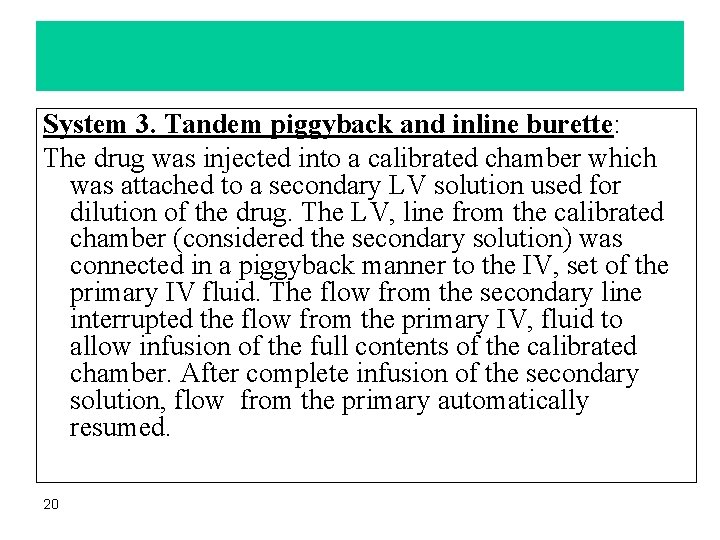
System 3. Tandem piggyback and inline burette: The drug was injected into a calibrated chamber which was attached to a secondary LV solution used for dilution of the drug. The LV, line from the calibrated chamber (considered the secondary solution) was connected in a piggyback manner to the IV, set of the primary IV fluid. The flow from the secondary line interrupted the flow from the primary IV, fluid to allow infusion of the full contents of the calibrated chamber. After complete infusion of the secondary solution, flow from the primary automatically resumed. 20

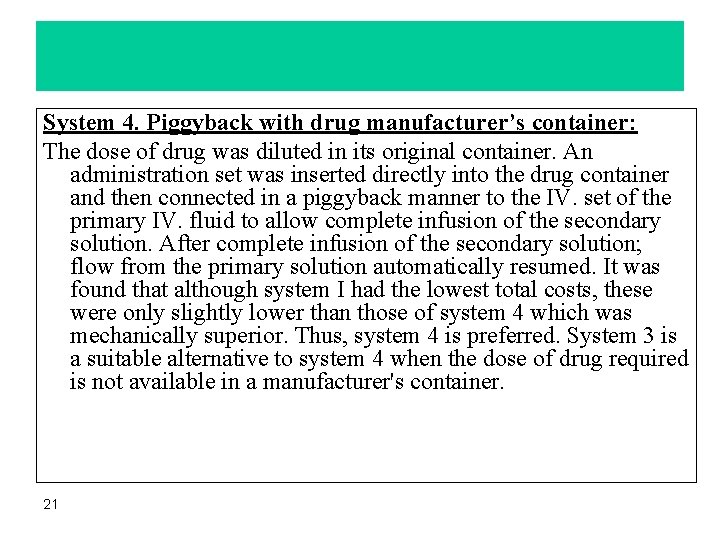
System 4. Piggyback with drug manufacturer’s container: The dose of drug was diluted in its original container. An administration set was inserted directly into the drug container and then connected in a piggyback manner to the IV. set of the primary IV. fluid to allow complete infusion of the secondary solution. After complete infusion of the secondary solution; flow from the primary solution automatically resumed. It was found that although system I had the lowest total costs, these were only slightly lower than those of system 4 which was mechanically superior. Thus, system 4 is preferred. System 3 is a suitable alternative to system 4 when the dose of drug required is not available in a manufacturer's container. 21

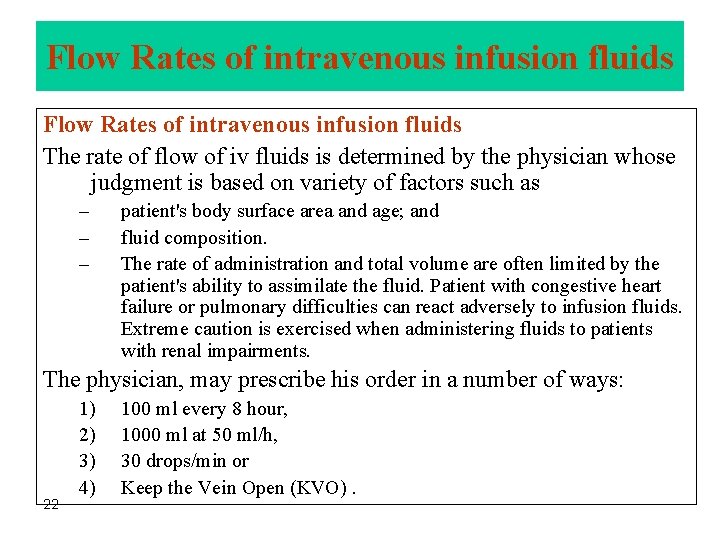
Flow Rates of intravenous infusion fluids The rate of flow of iv fluids is determined by the physician whose judgment is based on variety of factors such as – – – patient's body surface area and age; and fluid composition. The rate of administration and total volume are often limited by the patient's ability to assimilate the fluid. Patient with congestive heart failure or pulmonary difficulties can react adversely to infusion fluids. Extreme caution is exercised when administering fluids to patients with renal impairments. The physician, may prescribe his order in a number of ways: 22 1) 2) 3) 4) 100 ml every 8 hour, 1000 ml at 50 ml/h, 30 drops/min or Keep the Vein Open (KVO).

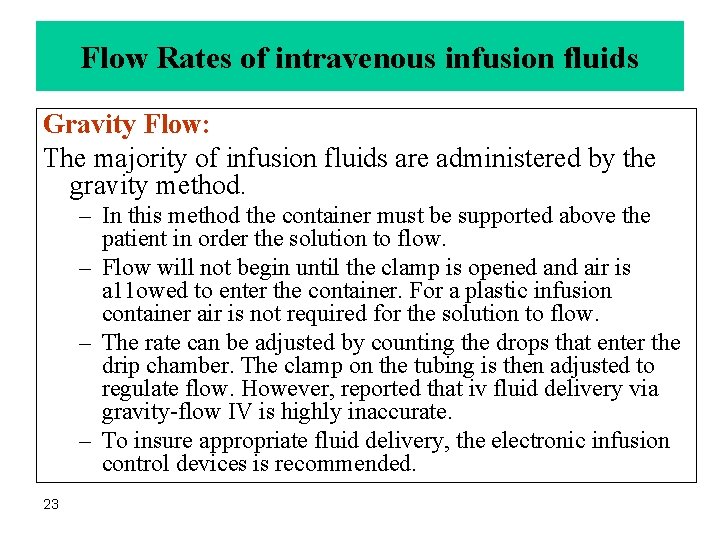
Flow Rates of intravenous infusion fluids Gravity Flow: The majority of infusion fluids are administered by the gravity method. – In this method the container must be supported above the patient in order the solution to flow. – Flow will not begin until the clamp is opened and air is a 11 owed to enter the container. For a plastic infusion container air is not required for the solution to flow. – The rate can be adjusted by counting the drops that enter the drip chamber. The clamp on the tubing is then adjusted to regulate flow. However, reported that iv fluid delivery via gravity-flow IV is highly inaccurate. – To insure appropriate fluid delivery, the electronic infusion control devices is recommended. 23

Flow Rates of intravenous infusion fluids Pumps and controllers: There are different types of pumps such as syringe pumps, peristaltic pumps and volumetric pumps. Volumetric pumps will be used as an aid for the infusion of the following types of solutions: parenteral nutrient: low-dose insulin infusion, lidocaine drips; dopamine, heparin infusion, intravenous fats, small volume administration, nitroprusside, magnesium infusion, blood (emergency only) and elemental diets. . 24

Techniques for preparation of admixtures: Admixture preparation requires strict attention to the three factors viz – maintenance of sterility, – avoiding particulate contamination and – prevention of incompatibility. a) Maintenance of sterility: The National Coordinating Committee on Large Volume parenteral (NCCLVP) recommended – the- availability of clearn work area with laminar air flow hood (class 100). – correct aseptic technique to be practiced and correct use of hood. The use of laminar flow work area incorporating a HEPA filter. Prefiltered air delivered under pressure passes in a uniform manner through the HEPA filter which filters about 99. 79% or all particles 0. 3 microns or larger, which includes airborne bacteria. The flow of air may be in either a horizontal or vertical pattern. 25

b) Avoiding particulate contamination: iv therapy provides direct access to a patients blood stream, the hazard of introducing particulate matter and microbial contamination by this route presents a serious problem. Particulate matter has been defined as the mobile undissolved substances present in parenteral product. It can include rubber, glass, metal, plastic or almost any substance. As with bacterial contamination, such particles can be introduced into iv fluids and drug containers at the time of manufacture or during preparation and use. Hence the use of inline filters in the IV administration system has been suggested to reduce the complication of infection and increase patient safety during long term IV therapy. A group of researchers studied the effect of filtration on the particulate matter and drug concentrations of solutions of cefazolin sodium in 0. 9% sodium chloride and dextrose 5% in water. They found that the p. H of the admixtures did not change over 24 hours and also no change of cefazolin concentration of unfiltered and filtered samples. 26

b) Avoiding particulate contamination: Addition of cefazolin to IV. infusion fluids increased particulate levels But after filtration particle counts of the DSW admixture were not significantly different than those of the infusion fluid. It was found that reconstituted amphotericin B "solutions" contain large numbers of particles less than 3 µm in size. Filtrations through a membrane filters were studied: It was found that filtration did not reduce the in vitro antimicrobial activity or alter the concentration of the drug. 27

c) Compatibility and stability: The compatibility of injectable drug admixtures is an area in which pharmacists can use their knowledge. The increasing number of injectable drugs and solutions increases the possibility of incompatibilities. Incompatibilities can be divided into three categories: therapeutic, physical and chemical. Therapeutic incompatibilities occur when two or more drugs are administered concurrently in antagonistic or synergistic pharmacology action. Physical incompatibility occurs when the combination of two or more drugs in solution results in a change in the appearance of the solution. However, degradation of drugs in solution resulting from the combination of parenteral dosage forms is called "chemical incompatibility". 28

Reports concerning the compatibility of ranitidine admixtures have been published. Lapason and coworkers concluded that ranitidine Hcl in concentration of 0. 5, 1. 0 and 2. 0 mg/ml in 5% dextrose injection or 0. 9% sodium chloride injection may be stored in polyvinyl chloride minibags frozen for 30 days followed by refrigeration for an additional 14 days. However, Stewart and others studied the stability of the same drug (0. 5, 1. 0 and 2. 0 mg/ml) in admixtures with commonly used IV. Fluid at different temperature conditions the admixture vehicles were: 0. 9% sodium chloride, 5% dextrose, 10% dextrose, 5% dextrose and 45% sodium chloride, and 5% dextrose with located 'Ringer's (DLR) injections in polyvinyl chloride bags. Their study revealed that Ranitidine is stable for 4 days at room temperature and 30 days at 4 -degrees C at all concentrations and in all vehicles studied. At the studied concentrations the drug is stable in admixtures frozen for 60 days and stored for seven days at room temperature or 14 days refrigerated, except in DLR admixtures, these admixtures should not be stored frozen. 29

Researchers studied the stability of intravenous admixtures of aztreonam and clindamycin phosphate using the same plan of study" Their results revealed that intravenous admixtures of both drugs at the concentrations studied are stable for at least 48 hours at 23°C and at least seven days at 4°C. Riley and Lipford recommended that aztreonam and nafcillin sodium should be administered separately because these drugs interact together in intravenous admixtures. The authors observed that when aztreonam and nafcillin sodium are mixed with 0. 9% sodium chloride injection or 5% dextrose injection in glass or plastic containers, a precipitate gradually forms. In 1978 Ludwig and Ueda investigated the apparent stability of nitroglycerin in dextrose 5% in water using both glass bottles and plastic bags under the following conditions: room temperature and room lights, room temperature protected from room light and refrigeration (4 QC). There appeared to be no difference in the apparent rate of NTG disappearance when prepared in either glass bottles or plastic bags. Protection of the drug solution from room light had no, apparent effect on the disappearance pattern of NTG. 30

Furthermore. refr 1 geration of the NTG solutions appeared to increase the stability of the admixtures. They concluded that sorption onto the walls of the glass bottles and plastic bags may account for the observed disappearance of NTG admixtures in dextrose 5% in water. However Mcniff et al, reported that nitroglycerin was found to be stable for at least 70 days when stored in glass container, regardless of source diluent (5% dextrose in water or normal saline) and storage temperature. The loss of NTG from solution, after storage for seven days in plastic IV bags, was greater when stored at room temperature (55%) than when refrigerated (30%). Adsorption was shown to be responsible for this phenomenon. 31

32