Intramolecular Bonding vs Intermolecular ForcesInteractions Intra 3 types
Intramolecular Bonding vs Intermolecular Forces/Interactions • Intra (3 types) • Inter (4 types) • Which is stronger (generally)? • Which determines state of matter?
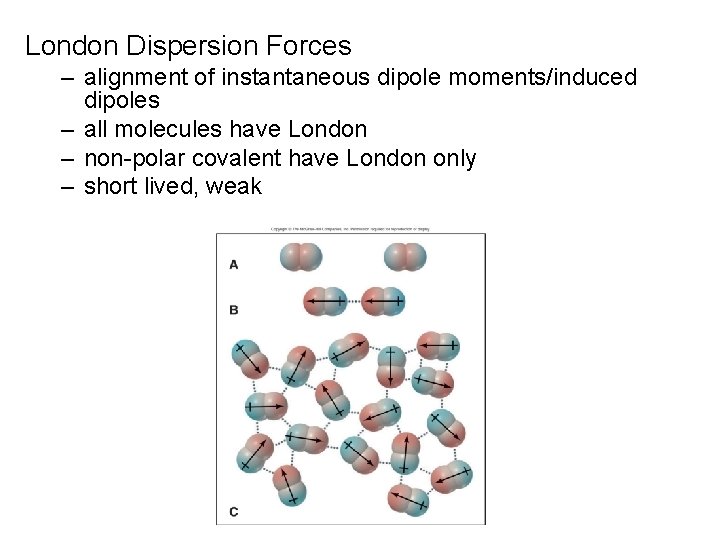
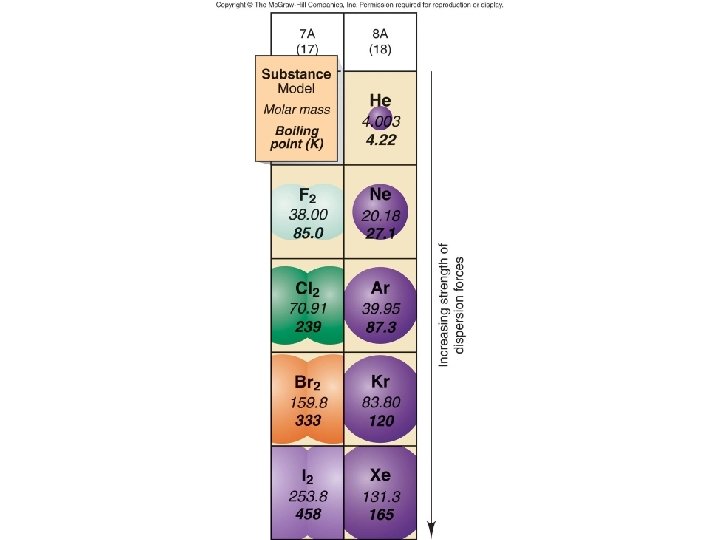
London Dispersion Forces – alignment of instantaneous dipole moments/induced dipoles – all molecules have London – non-polar covalent have London only – short lived, weak
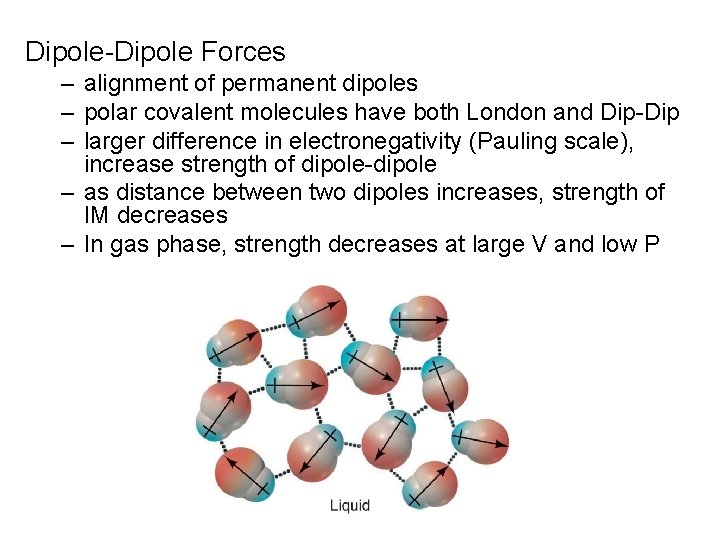
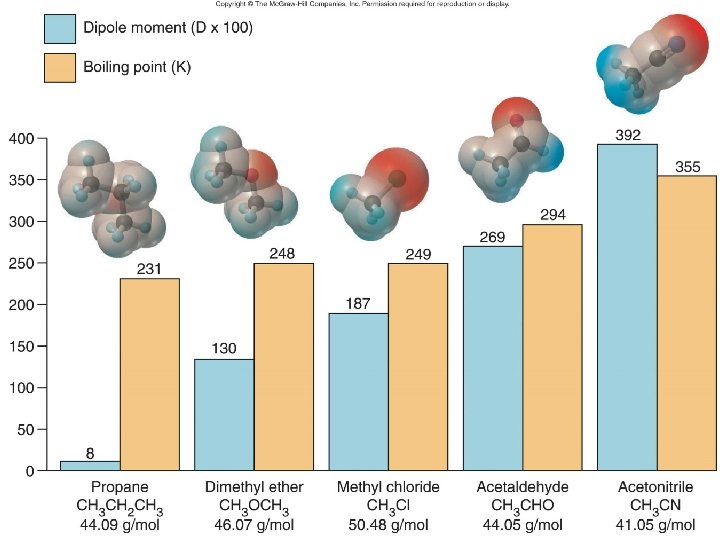
Dipole-Dipole Forces – alignment of permanent dipoles – polar covalent molecules have both London and Dip-Dip – larger difference in electronegativity (Pauling scale), increase strength of dipole-dipole – as distance between two dipoles increases, strength of IM decreases – In gas phase, strength decreases at large V and low P

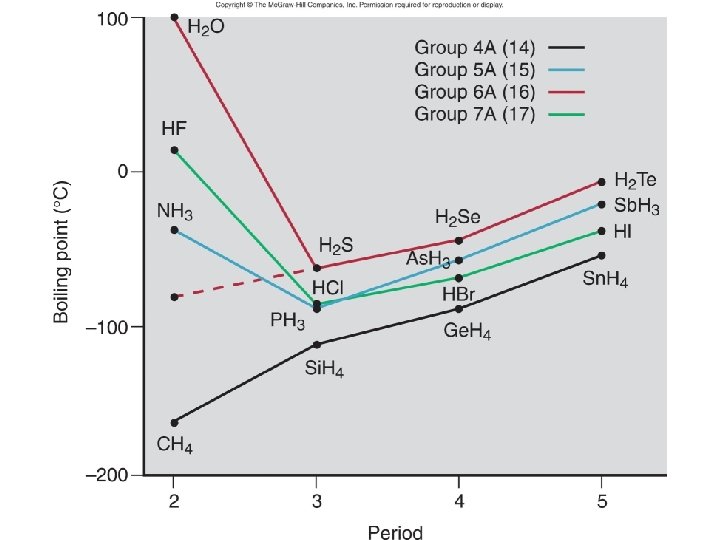
• H bonding – strongest of three IM forces we have discussed – Need H attached to O, N, F – We will call one molecule the H-donor; the other will be the H-acceptor • if you have both, you can H bond to yourself • Can water H bond to HF? HCN? • Can HCN H bond to itself? • Can acetone (CH 3 COCH 3) H bond to H 2 O? To itself?

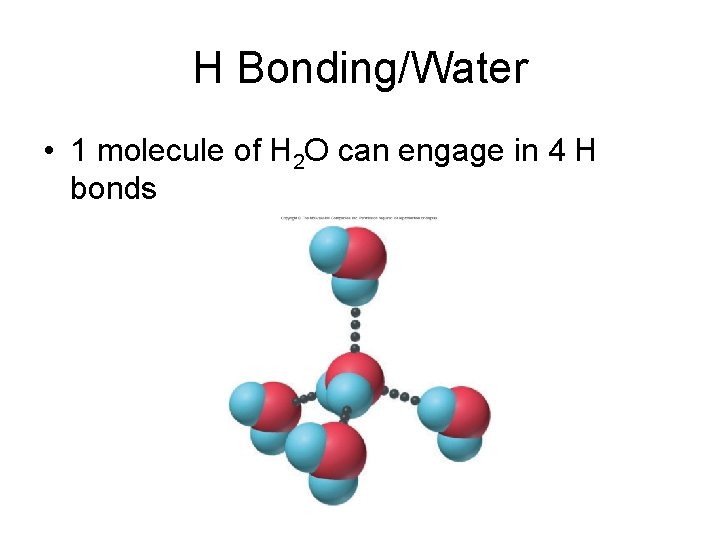
H Bonding/Water • 1 molecule of H 2 O can engage in 4 H bonds
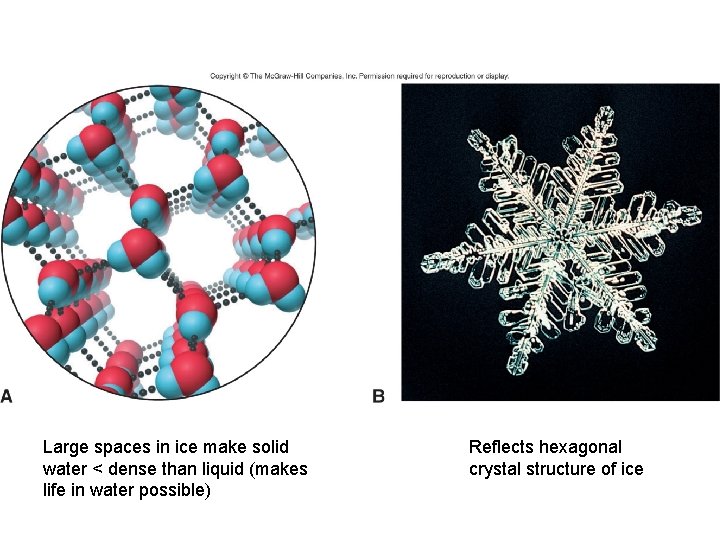
Large spaces in ice make solid water < dense than liquid (makes life in water possible) Reflects hexagonal crystal structure of ice

Ion Dipole Interactions – Generally, strongest of the four – When an ion “talks to” dipole moment – Ex. Na+ (aq) or Cl- (aq) Draw this interaction

Solvent Properties of Water (likes dissolve likes) • Can dissolve np, polar, ionic, and things that can H bond – Dissolves ionic compounds through ion-dipole forces – Dissolve many polar non-ionic compounds through H bonding or dipole-dipole – Dissolved non-polar gases through dipoleinduced dipole interactions
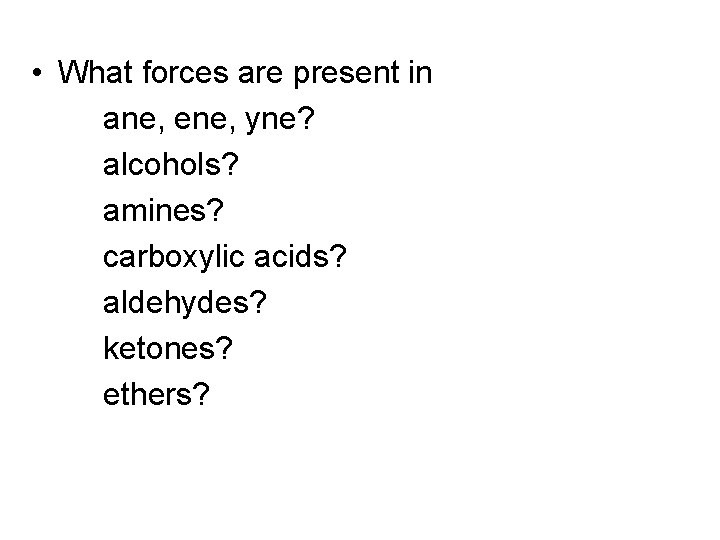
• What forces are present in ane, ene, yne? alcohols? amines? carboxylic acids? aldehydes? ketones? ethers?
• Which of the following has the strongest IM Forces? C 2 H 6 or C 2 H 5 Cl or C 2 H 5 OH CH 4 or C 2 H 6
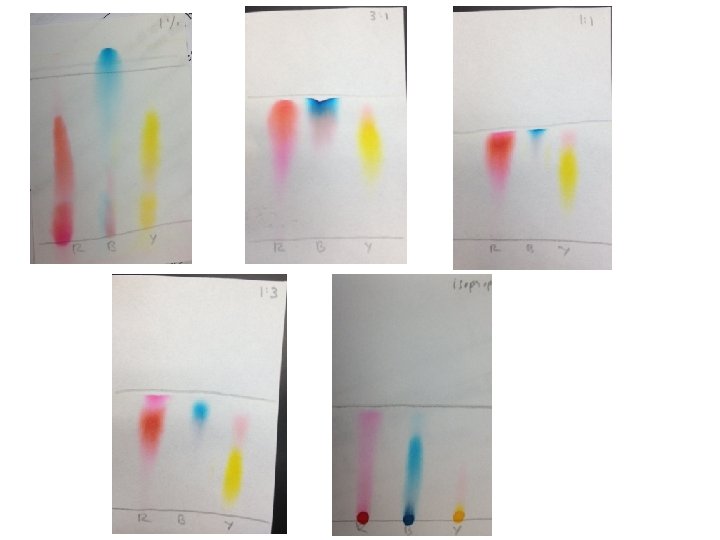
Chromatography HPLC, GLC, Paper, Column • Uses IM forces and solubility to separate components of mixture • Do this with two phases – Stationary Phase • medium you put the mixture on (filter paper) – Mobile Phase • put stationary phase in developing solvent. • As solvent moves along the stationary phase, create mobile phase.
Chromatography Separation is based upon differences in solubility or attraction to mobile phase versus the stationary phase If component is attracted to solvent, will move with mobile phase more than something that is attracted to stationary phase
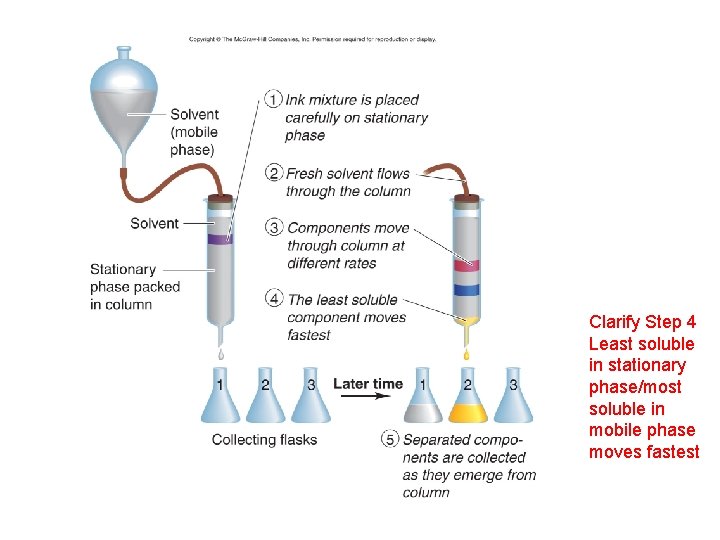
Clarify Step 4 Least soluble in stationary phase/most soluble in mobile phase moves fastest
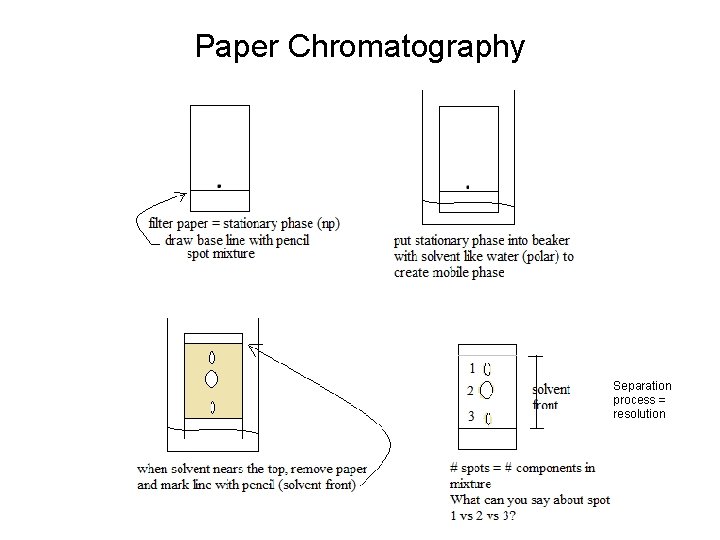
Paper Chromatography Separation process = resolution
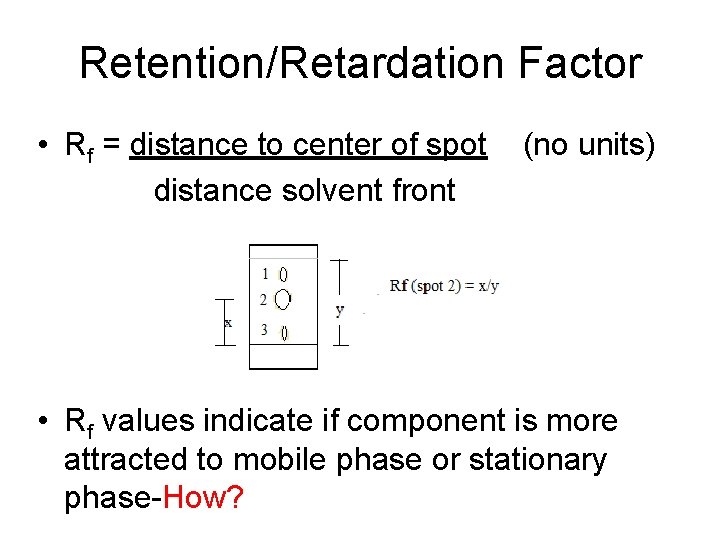
Retention/Retardation Factor • Rf = distance to center of spot distance solvent front (no units) • Rf values indicate if component is more attracted to mobile phase or stationary phase-How?


Physical properties like bp, surface tension, viscosity, and Pvap depend on – Size of molecule, MM • Generally, as increase MM, increase the physical properties – bp gets harder (requires higher temp), higher surface tension and more viscous – Strength of IM forces (depends on type)
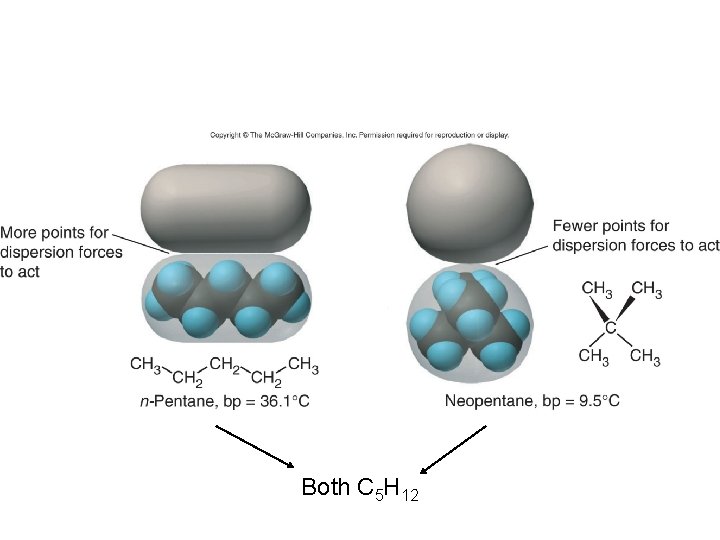
Both C 5 H 12
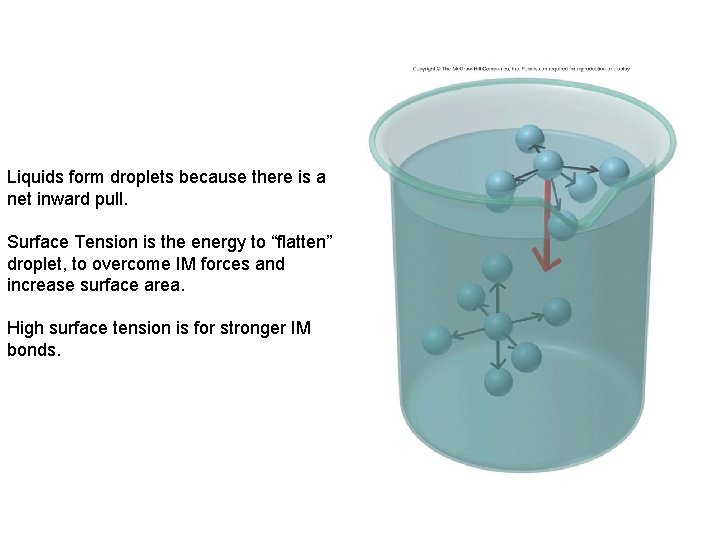
Liquids form droplets because there is a net inward pull. Surface Tension is the energy to “flatten” droplet, to overcome IM forces and increase surface area. High surface tension is for stronger IM bonds.

• Liquids with high surface tension have strong IM forces – Explains why bugs can walk on water but we cannot • Would you expect a higher surface tension for Ether or Methanol Methane or Ethane

Viscosity • Measure of a liquids resistance to flow (thickness) • Influenced by – – IM forces MM Shape (long chains make more contact) Temperature (hot oil flows more easily) Which has greater viscosity? Ethanol or methanol Butane or octane Butane or cyclobutane Butane or chlorobutane
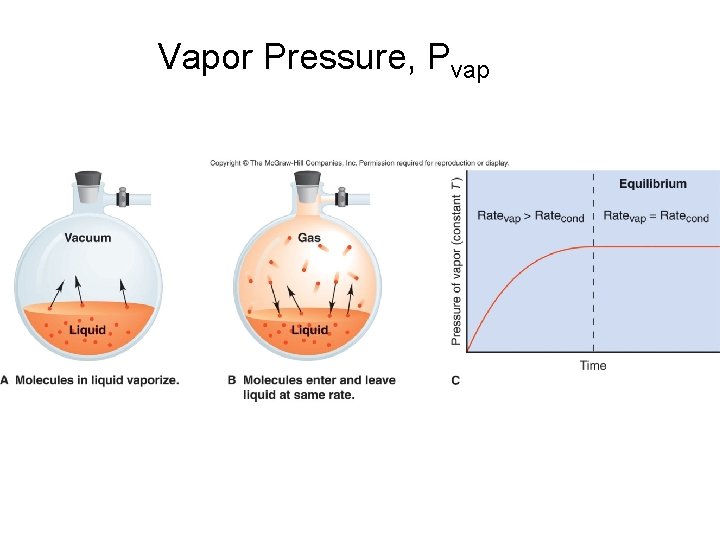
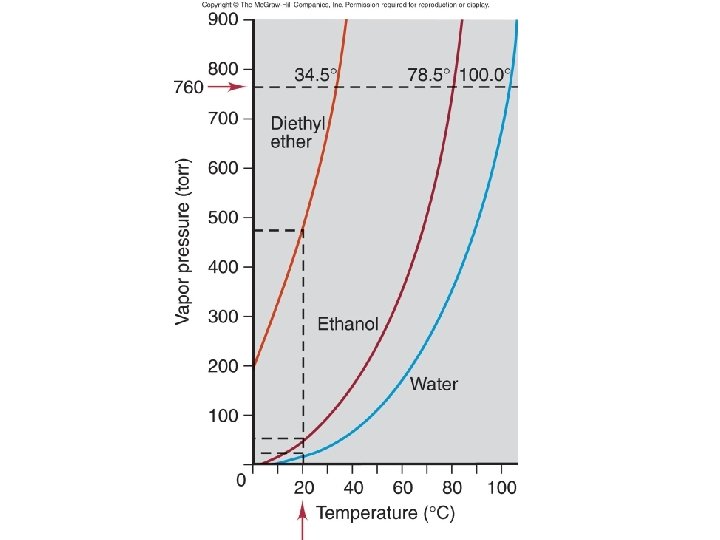
Vapor Pressure, Pvap
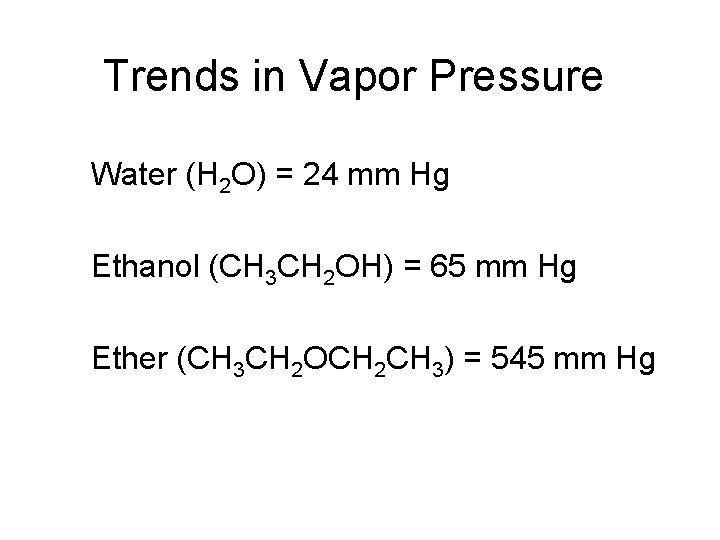
Trends in Vapor Pressure Water (H 2 O) = 24 mm Hg Ethanol (CH 3 CH 2 OH) = 65 mm Hg Ether (CH 3 CH 2 OCH 2 CH 3) = 545 mm Hg

Vapor Pressure affected by – Strength of IM forces – Size of molecule (MM) – Surface area What about temperature?
- Slides: 31