Intramolecular and Intermolecular Forces Week 4 Tuesday Electronegativity
































- Slides: 54

Intramolecular and Intermolecular Forces Week 4 Tuesday

Electronegativity A measure of an atoms ability to attract the pair of electrons that it shares with another atom with in a covalent bond.

Electronegativity The atomic radius plays a big role in how electronegative an atom will be. The larger an atom is the weaker the attraction for shared electron pairs will be. This is due to electrons shielding each other and thus making the attraction of electron pairs less.

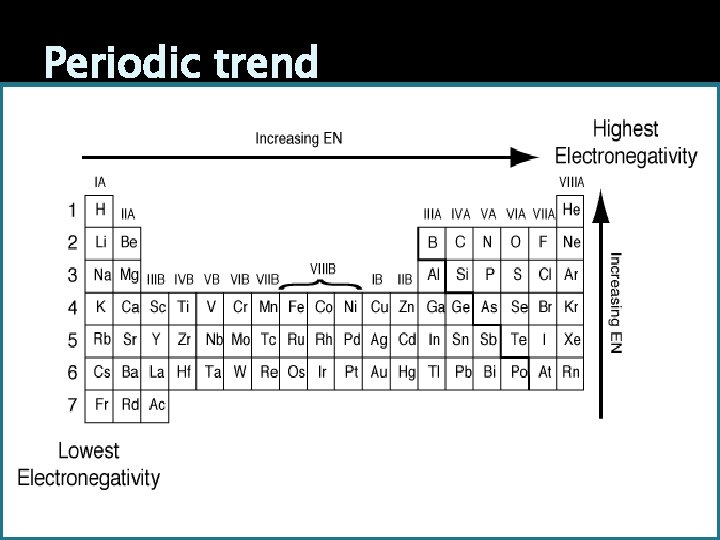
Periodic trend As the number of shells increase electronegativity decreases (move down the periodic table) As more protons are added atomic radius decreases and thus increases electronegativity. (move left to right)

Periodic trend

Polar and Nonpolar Covalent Bonds (p 41) Intramolecular Force - the attractive force between atoms and ions in a compound If the electron pair is shared in an equal manner (ie. Each atom has an equal attraction to the shared electron pair), then the bond is a nonpolar covalent bond. Ex H 2, O 2, Cl 2.

Polar and Nonpolar Covalent Bonds (p 41) When electron pairs are not shared equally between atoms they will spend more time near one atom than another. This would mean that one of the atoms is slightly more negative than the other atom. To show these partial localized charges, we use the symbol δ+ for a localized positive charge and δ- for a localized negative charge. These bonds are called polar covalent bonds. Example HCl.

Whether or not a bond is polar covalent depends on the difference between the electronegativities of the bonded atoms. The larger the difference in electronegativities the more polar the bond is. A bond is considered ionic when the difference is 1. 7. Some ionic bonds will have less than this.

Polar and Non-polar molecules (p 42) Polar molecules - are molecules that have a positive charged end a negative charged end. Nonpolar molecules - are molecules that do not have charged ends.

Polar and nonpolar molecules The polarity of a molecule depends on two characteristics of the molecule. 1. The presence of polar covalent bonds; 2. The three dimensional shape (geometry) of the molecule

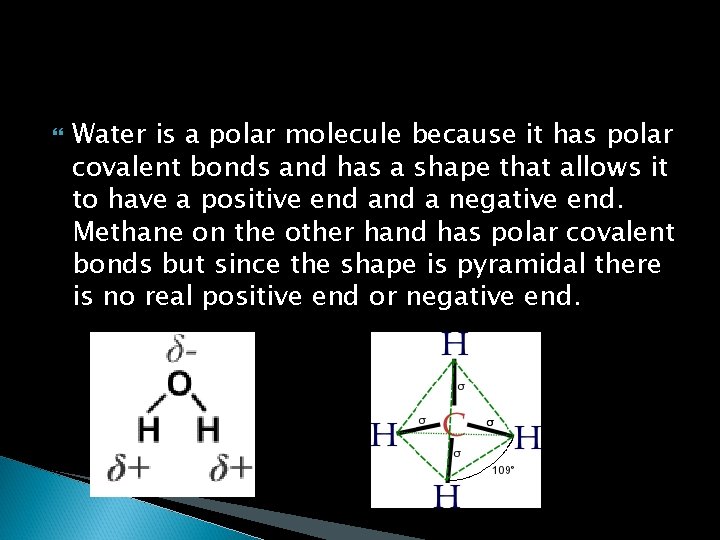
Water is a polar molecule because it has polar covalent bonds and has a shape that allows it to have a positive end a negative end. Methane on the other hand has polar covalent bonds but since the shape is pyramidal there is no real positive end or negative end.

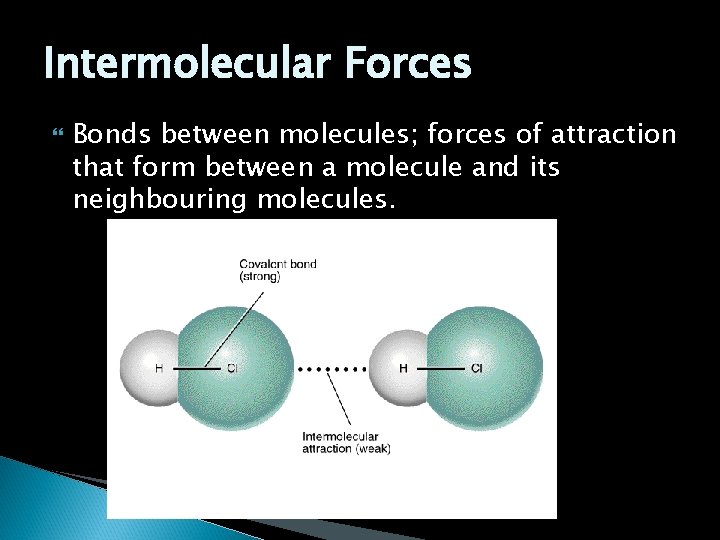
Intermolecular Forces Bonds between molecules; forces of attraction that form between a molecule and its neighbouring molecules.

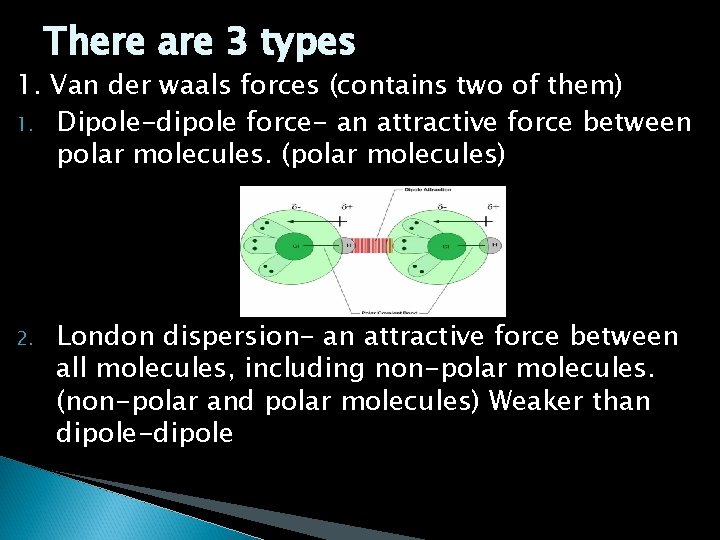
There are 3 types 1. Van der waals forces (contains two of them) 1. Dipole-dipole force- an attractive force between polar molecules. (polar molecules) 2. London dispersion- an attractive force between all molecules, including non-polar molecules. (non-polar and polar molecules) Weaker than dipole-dipole

2. Hydrogen bonding – Similar to dipole-dipole but stronger. It occurs in highly polar molecules have either a H-N, H-O or H-F bond. This is why it requires large amounts of energy to break water molecules apart. (solid, liquid) -When water freezes it forms a lattice structure which makes it less dense than water.


Homework Try this (p 44) – if in class with model kit Page 45 # 1 -6

Molecular Nomenclature

Molecular Nomenclature The names of molecular compounds often contain prefixes. The prefixes are used to count the number of atoms in the molecule. This is important as molecular compounds containing 2 elements can have different combinations which have different properties.

Binary Molecular Compounds: IUPAC 1. Write down the name of the first element. If there is more than one atom of this element attach a Greek prefix. (if there is only one atom do not attach the prefix) 2. Attach a Greek prefix (relating to the number of atoms) to the second elements name and add -ide. ◦ Example: CO = Carbon monoxide CO 2 = Carbon dioxide

Prefixes used when naming binary molecular compounds # of atoms 1 Prefix mono 2 3 4 5 6 7 8 9 10 di tri tetra penta hexa hepta octa nona deca

Practice HF = PBr = CS 2 = NO = H 2 S = NO 2 = SCl 2 = N 2 O 2 = sulphur trioxide = dihydrogen monoxide = carbon tetrafluoride = Silicon dibromide = Trihydrogen mononitride = Disulfur trioxide = Carbon diphosphide = Chlorine gas =

Word Equations

Word Equations A word equation is a way of representing a chemical reaction: it tells you what reacts and what is produced. Word equations are an efficient way to describe chemical changes, to help chemists recognise patterns, and to predict the products of a chemical reaction.

Word Equations They are written in a particular order. Reactants are always on the left side of the arrow and products are always on the right side of the arrow. Multiple reactants or products are separated by a + sign.

Examples Silver nitrate + copper silver + copper(II) nitrate Hydrogen + Oxygen water vapour Which are the products and which are reactants

The Conservation of Mass

The Conservation of Mass In a chemical reaction, the total mass of the reactants is always equal to the total mass of the products. This tells us a few things.

Conservation of mass Atoms do not change in a reaction. The molecules that they form can be changed but the atoms themselves are not. Mass cannot be destroyed. If it could we could use E = MC 2

Example Methane + oxygen water + carbon dioxide

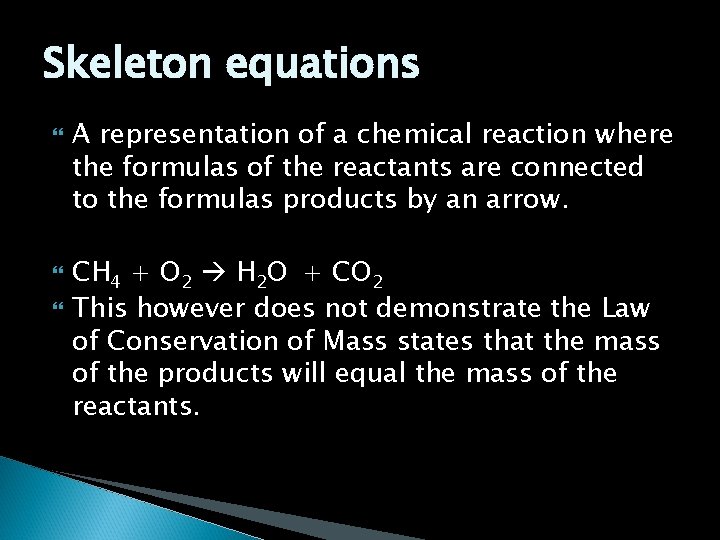
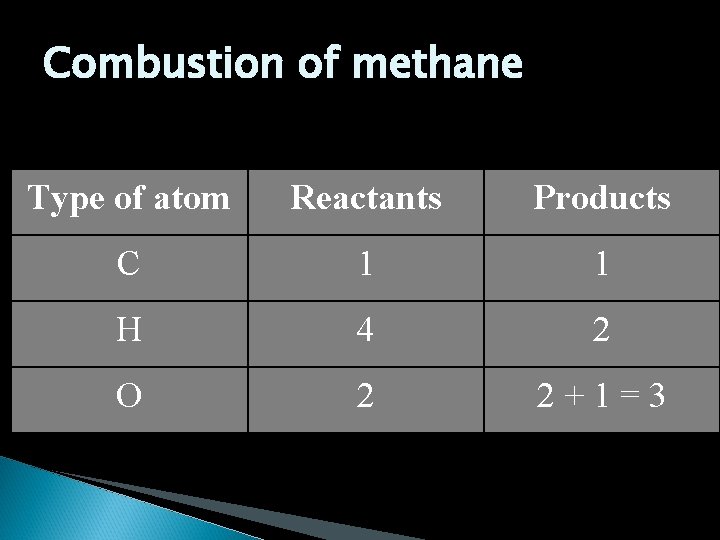
Skeleton equations A representation of a chemical reaction where the formulas of the reactants are connected to the formulas products by an arrow. CH 4 + O 2 H 2 O + CO 2 This however does not demonstrate the Law of Conservation of Mass states that the mass of the products will equal the mass of the reactants.

Combustion of methane Type of atom Reactants Products C 1 1 H 4 2 O 2 2+1=3

We can’t change the formulas of the products or reactants so the only thing we can do is change the number of molecules instead of their formulas.

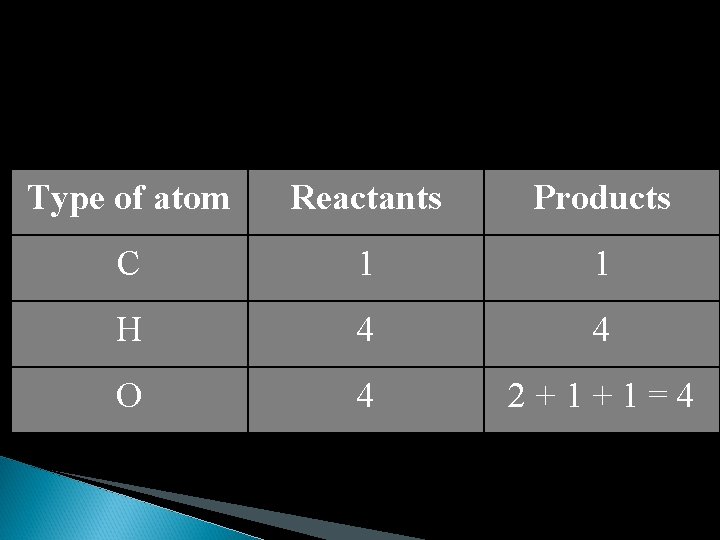
CH 4 + O 2 H 2 O + CO 2 = CH 4 + 2 O 2 2 H 2 O + CO 2 Now the chemical equation is balanced and the mass of the reactants will equal the mass of the products

Type of atom Reactants Products C 1 1 H 4 4 O 4 2+1+1=4

Steps to balancing an equation Step 1 Write the word equation of the reaction Aluminum + bromine aluminum bromide Step 2 Write the skeleton equation by replacing each name with a correct formula. Al +Br 2 Al. Br 3

Step 3 Type of atom Al Br Reactants Products 1 2 1 3

Step 4 Multiply each of the formulas by the appropriate coefficients to balance the number of atoms. Start out by picking the element with the most number of atoms and try to balance it first. We will start with Bromine. The 2 and 3 will be balanced if we multiply the reactant side by 3 which would give it 6 Br, and multiply the product side by 2 to give us 6 Br. Now we have 2 Al products which need to be balanced so we add a 2 to the Al on the reactant side. 2 Al + 3 Br 2 2 Al. Br 3

More examples Hydrogen gas + Chlorine gas hydrogen chloride Sodium + chlorine sodium chloride Nitrogen + hydrogen ammonia (hydrogen nitride) N 2 + H 2 NH 3 = N 2 + H 2 NH 3

Types of chemical reactions (P 48 -52)

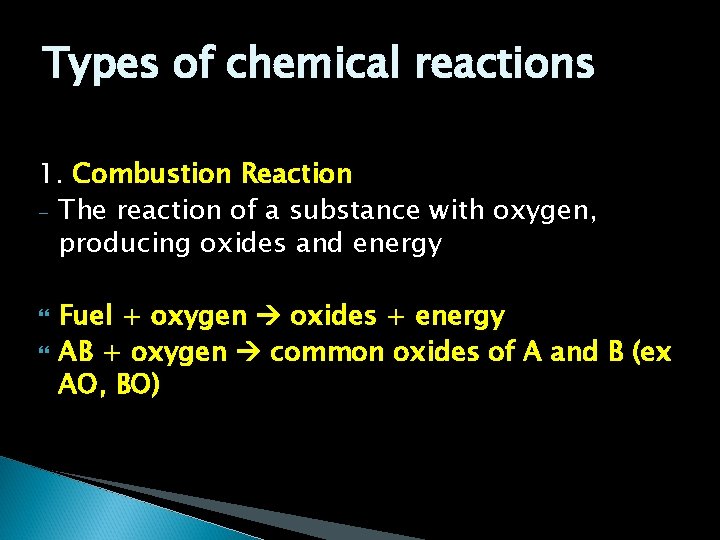
Types of chemical reactions 1. Combustion Reaction - The reaction of a substance with oxygen, producing oxides and energy Fuel + oxygen oxides + energy AB + oxygen common oxides of A and B (ex AO, BO)

Common oxides include C = CO 2(g) H = H 2 O(g) S = SO 2(g) N =NO 2(g)

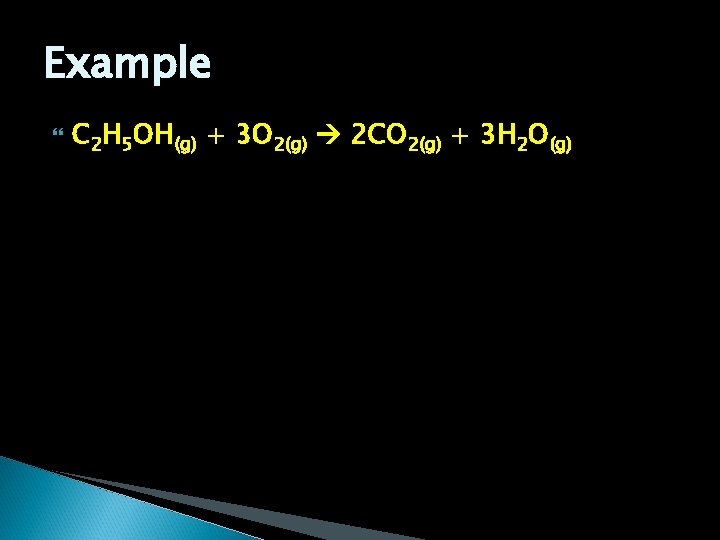
Example C 2 H 5 OH(g) + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(g)

Incomplete combustion occurs when there is not sufficient oxygen. When this occurs 4 products are produced instead of the usual H 2 O and CO 2. Incomplete combustion also produces CO(g) and C(s). This is commonly seen when lighting an acetylene torch. 3 C 2 H 2 (g) + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(g) + 2 C(s)

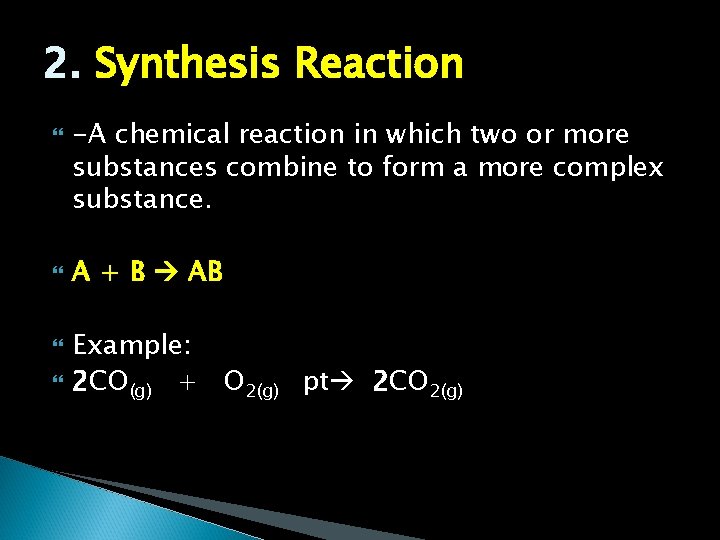
2. Synthesis Reaction -A chemical reaction in which two or more substances combine to form a more complex substance. A + B AB Example: 2 CO(g) + O 2(g) pt 2 CO 2(g)

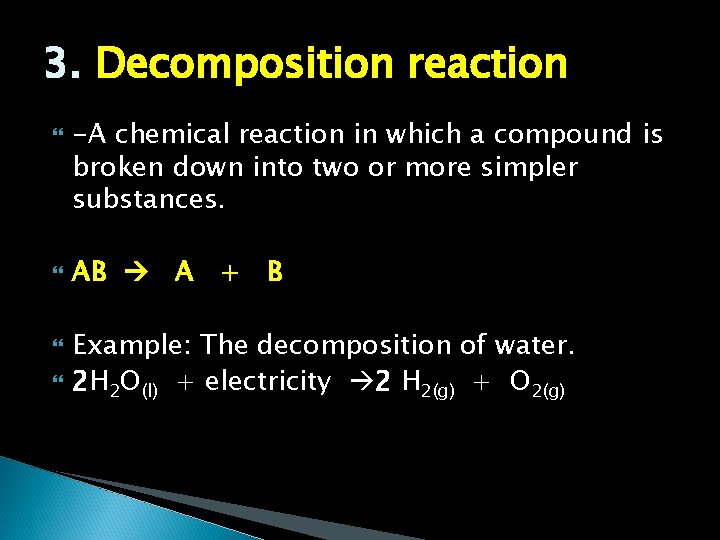
3. Decomposition reaction -A chemical reaction in which a compound is broken down into two or more simpler substances. AB A + B Example: The decomposition of water. 2 H 2 O(l) + electricity 2 H 2(g) + O 2(g)

Chemical reactions in solution A Solution is a homogenous mixture in which a pure substance, called the solute, is dissolved in another pure substance called the solvent. The solution is often an aqueous solution which is a solution where water is the solvent.

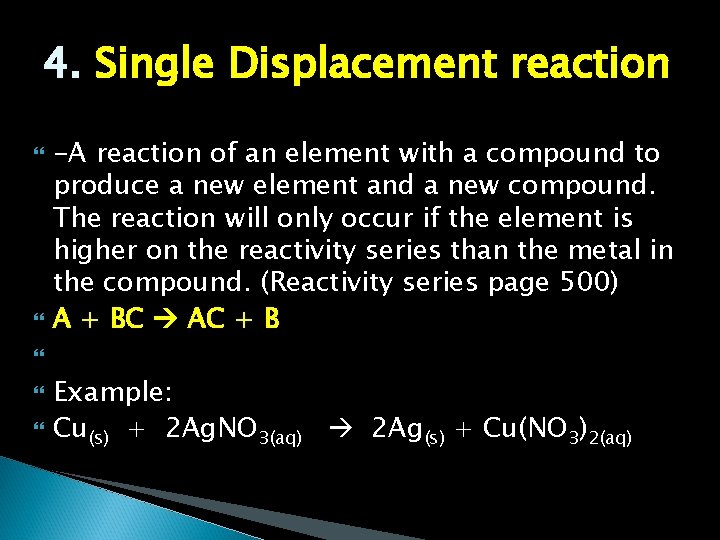
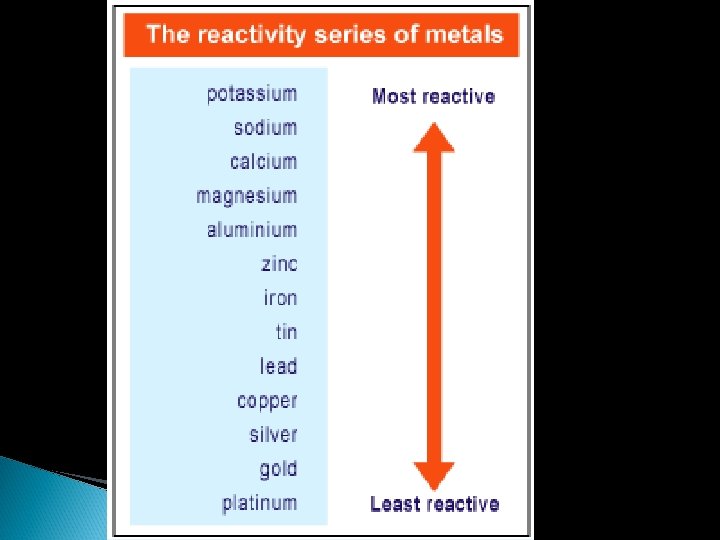
4. Single Displacement reaction -A reaction of an element with a compound to produce a new element and a new compound. The reaction will only occur if the element is higher on the reactivity series than the metal in the compound. (Reactivity series page 500) A + BC AC + B Example: Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq)


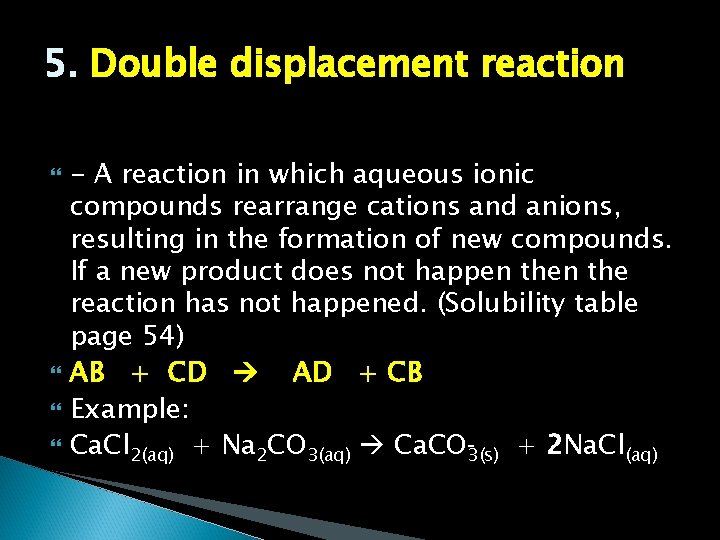
5. Double displacement reaction - A reaction in which aqueous ionic compounds rearrange cations and anions, resulting in the formation of new compounds. If a new product does not happen the reaction has not happened. (Solubility table page 54) AB + CD AD + CB Example: Ca. Cl 2(aq) + Na 2 CO 3(aq) Ca. CO 3(s) + 2 Na. Cl(aq)

Double displacement is likely to occur if; - a precipitate is produced - a gas is produced - a acid base neutralization occurs

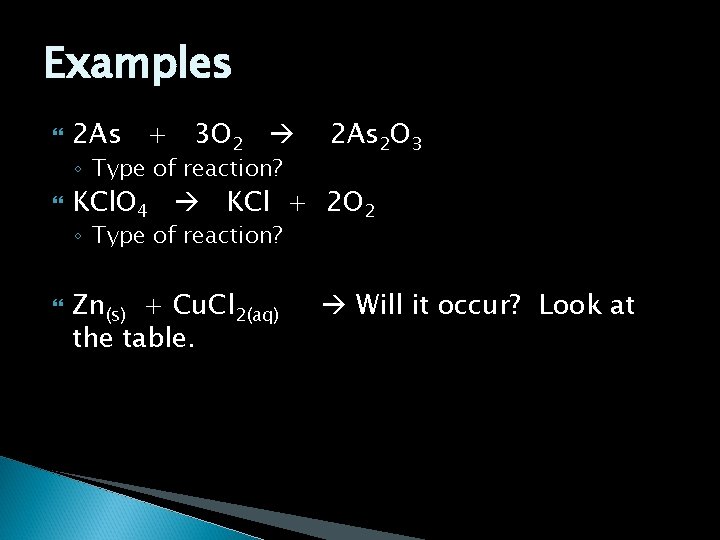
Examples 2 As + 3 O 2 ◦ Type of reaction? 2 As 2 O 3 KCl. O 4 KCl + 2 O 2 ◦ Type of reaction? Zn(s) + Cu. Cl 2(aq) the table. Will it occur? Look at

Cu(s) + Zn. Cl 2(s) will it occur Mg. Cl 2(aq) + K(OH)2(aq) will it occur? Name the precipitate

Work sheet This will be handed in for marks. Do it, it is great practice.

Review If you would like the review now, I can give you the paper copy with the practice questions.