Interstitial Lung Diseases ILD Diffuse Parenchymal Lung Diseases





















- Slides: 52

Interstitial Lung Diseases (ILD) Diffuse Parenchymal Lung Diseases (DPLD) Edit Csada, MD 10. 06. 2016. 1

Interstitial lung disorders (ILD) The interstitial lung disorders are chronic, nonmalignant, noninfectious diseases of the lower respiratory tract characterized by inflammation and derangement of the alveolar walls. n Secondary fibrosis and pulmonal hypertension may develop n Prevalence: 7 -20/100 000 n 2

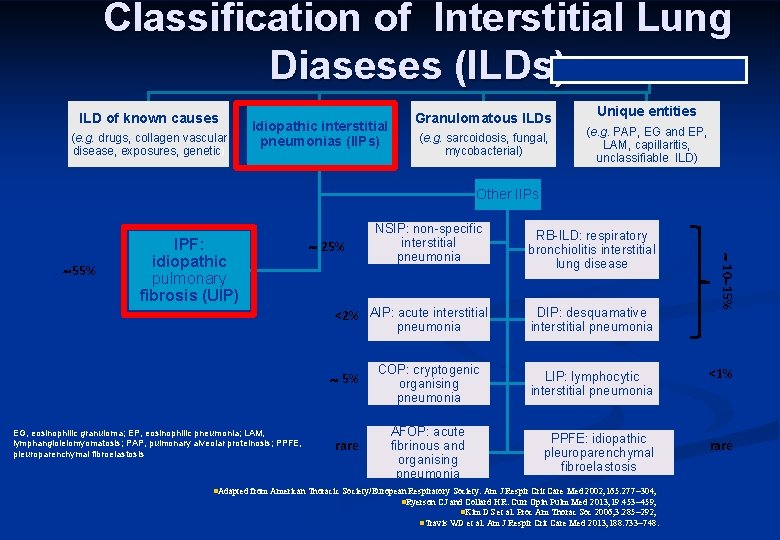
Classification of Interstitial Lung Diaseses (ILDs) ILD of known causes (e. g. drugs, collagen vascular disease, exposures, genetic) Idiopathic interstitial pneumonias (IIPs) Granulomatous ILDs (e. g. sarcoidosis, fungal, mycobacterial) Unique entities (e. g. PAP, EG and EP, LAM, capillaritis, unclassifiable ILD) Other IIPs 25% <2% AIP: acute interstitial pneumonia 5% EG, eosinophilic granuloma; EP, eosinophilic pneumonia; LAM, lymphangioleiomyomatosis; PAP, pulmonary alveolar proteinosis; PPFE, pleuroparenchymal fibroelastosis rare COP: cryptogenic organising pneumonia AFOP: acute fibrinous and organising pneumonia RB-ILD: respiratory bronchiolitis interstitial lung disease DIP: desquamative interstitial pneumonia 10 15% 55% IPF: idiopathic pulmonary fibrosis (UIP) NSIP: non-specific interstitial pneumonia LIP: lymphocytic interstitial pneumonia <1% PPFE: idiopathic pleuroparenchymal fibroelastosis rare n. Adapted from American Thoracic Society/European Respiratory Society. Am J Respir Crit Care Med 2002; 165: 277 304; n. Ryerson CJ and Collard HR. Curr Opin Pulm Med 2013; 19: 453 459; n. Kim DS et al. Proc Am Thorac Soc 2006; 3: 285 292; n. Travis WD et al. Am J Respir Crit Care Med 2013; 188: 733 748.

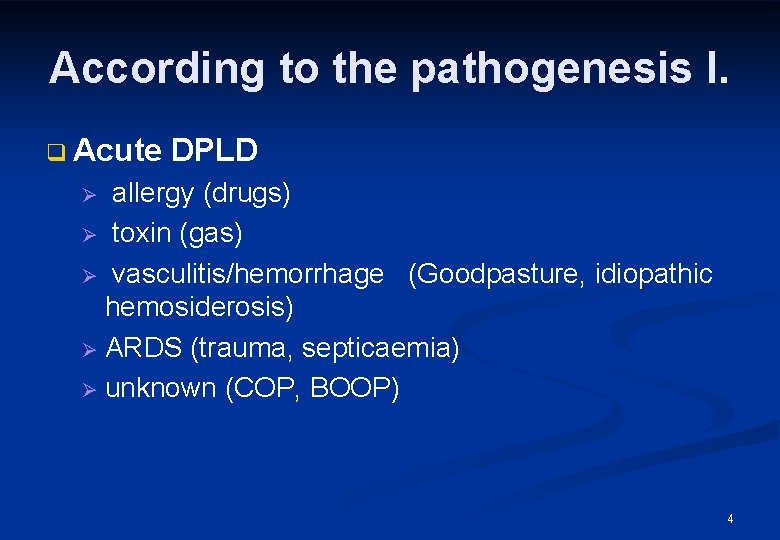
According to the pathogenesis I. q Acute DPLD allergy (drugs) Ø toxin (gas) Ø vasculitis/hemorrhage (Goodpasture, idiopathic hemosiderosis) Ø ARDS (trauma, septicaemia) Ø unknown (COP, BOOP) Ø 4

According to the pathogenesis II. n Episodic DPLD eo pneumonia Ø vasculitis, hemorrhage Ø Churg-Strauss sy Ø Hypersensitive pneumonitis Ø COP Ø 5

According to the pathogenesis III. n Chronic DPLD (exposition) inorganic dusts (silicosis, asbestosis) Ø organic dusts (bacteria, fungi, animal proteins) Ø drugs Ø 6

According to the pathogenesis IV. n Chronic DPLD (systemic diseases) Ø sarcoidosis connective tissue diseases Ø malignant diseases (lymphoma, lymphangitis cc) Ø vasculitis (Wegener) Ø hereditary diseases Ø others ( bone marrow transplantation, inflammatory intestine diseases, amyloidosis) Ø 7

According to the pathogenesis V. n Chronic DPLD (without systemic disease and without exposition) Idiopathic interstitial pneumonitis (IIP) Ø alveolar proteinosis Ø chronic aspiration Ø LAM Ø Langerhans-cell histiocytosis Ø venoocclusive disease Ø idiopathic pulmonal haemosiderosis Ø bronchioloalveolar cc. Ø 8

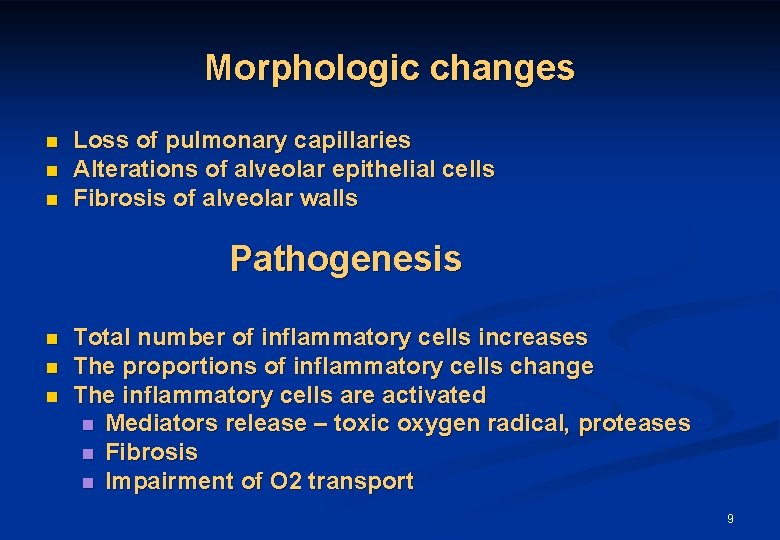
Morphologic changes n n n Loss of pulmonary capillaries Alterations of alveolar epithelial cells Fibrosis of alveolar walls Pathogenesis n n n Total number of inflammatory cells increases The proportions of inflammatory cells change The inflammatory cells are activated n Mediators release – toxic oxygen radical, proteases n Fibrosis n Impairment of O 2 transport 9

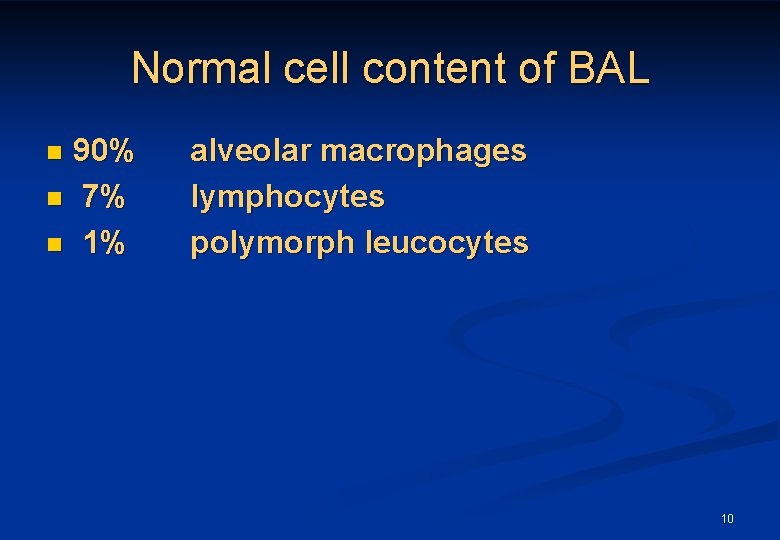
Normal cell content of BAL 90% n 7% n 1% n alveolar macrophages lymphocytes polymorph leucocytes 10

• Neutrophyl alveolitis : cryptogenic fibrotic alveolitis • Lymfocytic alveolitis : sarcoidosis, hypersensitive pneumonitis, beryllosis • Eosinophil alveolitis • Mixed-cell alveolitis: amiodaron fibrosis (eosinophil+lymphocyte) 11

Clinical features in ILD I. n Symptoms Dyspnea during exercise n Fatigue n Nonproductive cough n n Physical finding Dry, crackling rales n Wheezing n Tubular breath sounds n Digital clubbing n 12

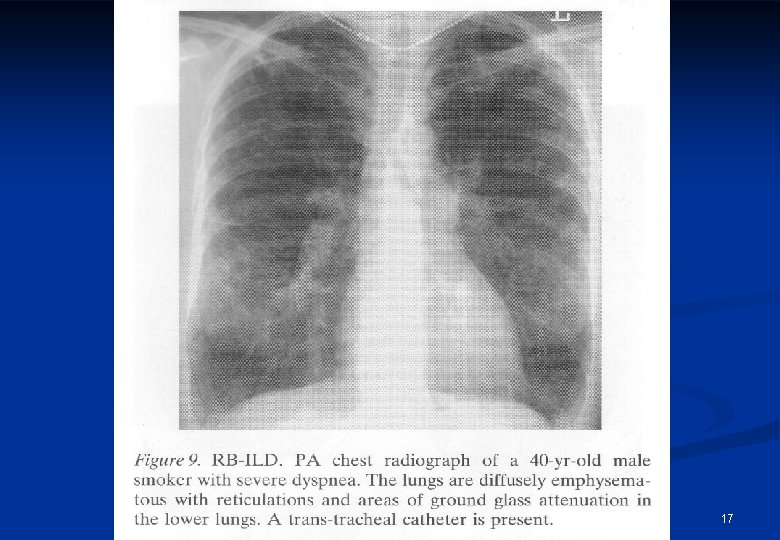
Clinical features in ILD II. n Laboratory changes ERS can be elevated n Hypoxaemia n n Chest X-ray, HRCT scan Reticular n Nodular n Reticulonodular n Ground glass haziness n Honeycombing n 13

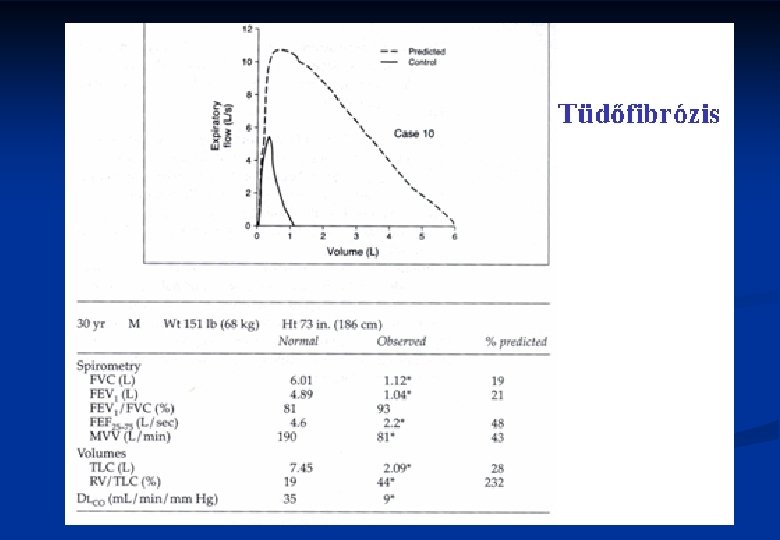
Clinical features in ILD III. n Lung function tests n n n Scintigraphic findings Tc 99, Xe 133 n n Reduction in VC, TLC FEV 1/FVC is normal, or supranormal Decrease in diffusing capacity, transfer factor (DLCO) Oxyergospirometry (exercise test) Patchy abnormalities BAL n Various mixtures of inflammatory cells 14

15

16

17

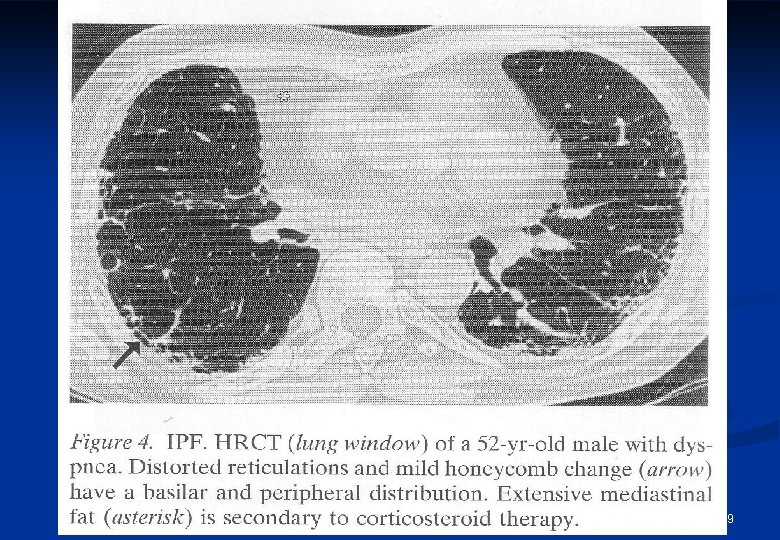
18

19

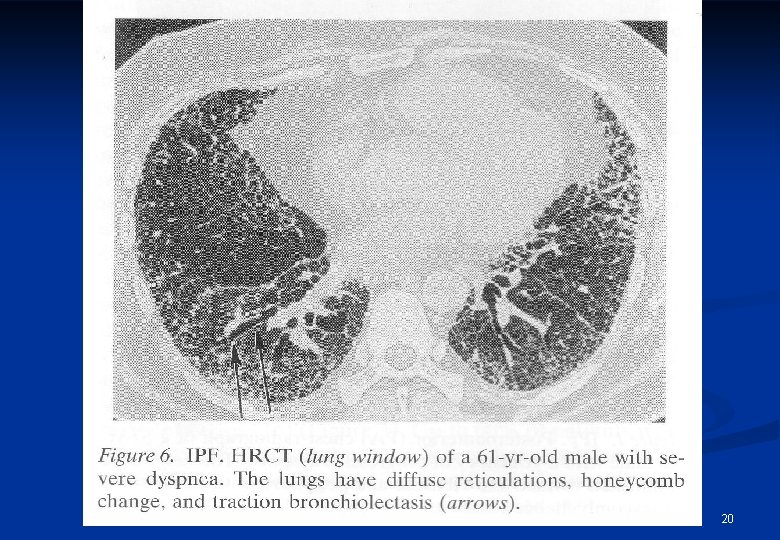
20

Diagnosis of ILD Clinical features n Transbronchial biopsy n VATS, open lung biopsy n 21

Therapy of ILD n n n Known etiology n Remove the individual from exposure to the causative agents Known and unknown etiology n Suppress inflammatory process n Oral corticosteroids (1 mg/kg 0, 25 mg/kg) n CPA (cyclophosphamide) n Imuran (azathioprine) Late stage n O 2 therapy n Supportive treatment n Transplantation 22

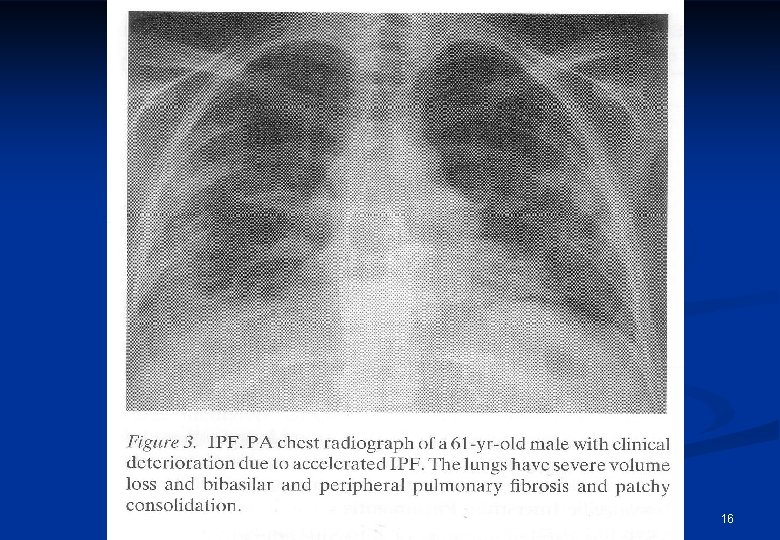
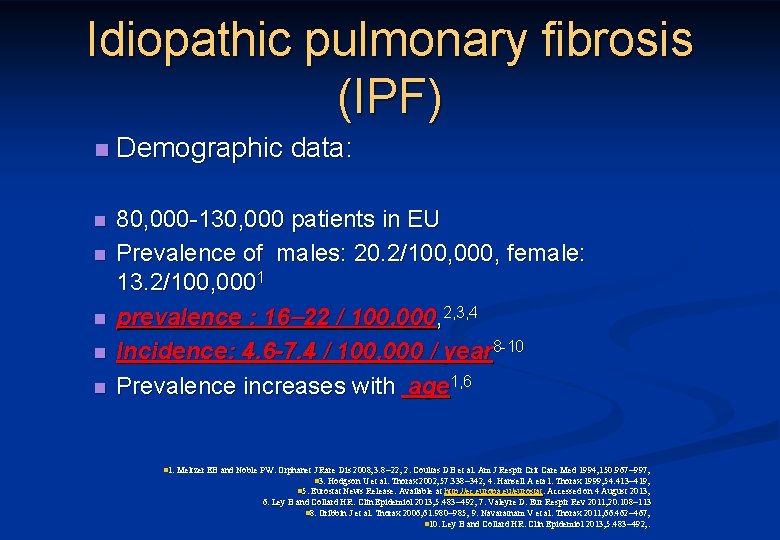
Idiopathic pulmonary fibrosis (IPF) n Demographic data: n 80, 000 -130, 000 patients in EU Prevalence of males: 20. 2/100, 000, female: 13. 2/100, 0001 prevalence : 16 22 / 100, 000, 2, 3, 4 Incidence: 4. 6 -7. 4 / 100, 000 / year 8 -10 Prevalence increases with age 1, 6 n n n 1. Meltzer EB and Noble PW. Orphanet J Rare Dis 2008; 3: 8 22; 2. Coultas DB et al. Am J Respir Crit Care Med 1994; 150: 967 997; n 3. Hodgson U et al. Thorax 2002; 57: 338 342; 4. Hansell A eta l. Thorax 1999; 54: 413 419; n 5. Eurostat News Release. Available at http: //ec. europa. eu/eurostat. Accessed on 4 August 2013; 6. Ley B and Collard HR. Clin Epidemiol 2013; 5: 483 492; 7. Valeyre D. Eur Respir Rev 2011; 20: 108 113 n 8. Gribbin J et al. Thorax 2006; 61: 980 985; 9. Navaratnam V et al. Thorax 2011; 66: 462 467; n 10. Ley B and Collard HR. Clin Epidemiol 2013; 5: 483 492; .

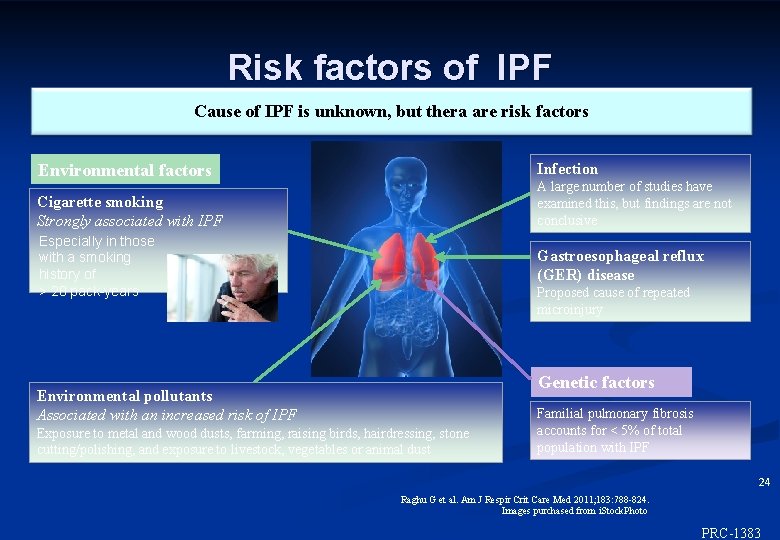
Risk factors of IPF Cause of IPF is unknown, but thera are risk factors Infection Environmental factors A large number of studies have examined this, but findings are not conclusive Cigarette smoking Strongly associated with IPF Especially in those with a smoking history of > 20 pack-years Gastroesophageal reflux (GER) disease Proposed cause of repeated microinjury Genetic factors Environmental pollutants Associated with an increased risk of IPF Exposure to metal and wood dusts, farming, raising birds, hairdressing, stone cutting/polishing, and exposure to livestock, vegetables or animal dust Familial pulmonary fibrosis accounts for < 5% of total population with IPF 24 Raghu G et al. Am J Respir Crit Care Med 2011; 183: 788 -824. Images purchased from i. Stock. Photo ©Inter. Mune, Inc. , 2012 PRC-1383

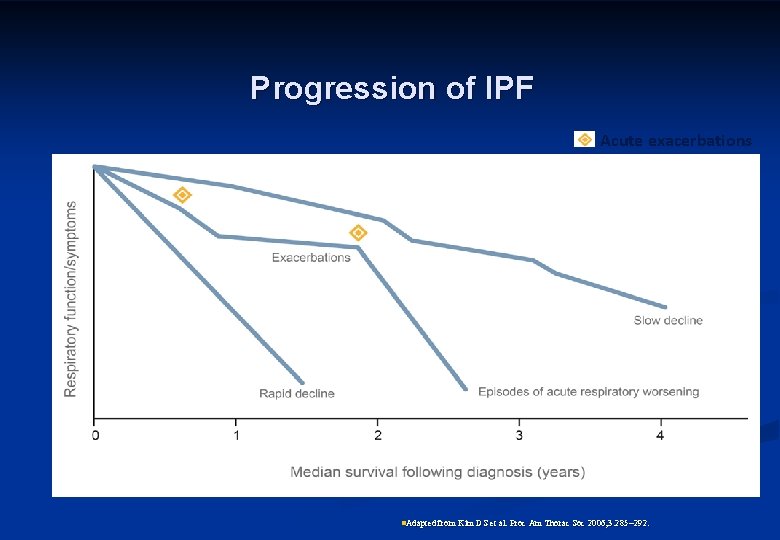
Progression of IPF Acute exacerbations n. Adapted from Kim DS et al. Proc Am Thorac Soc 2006; 3: 285 292.

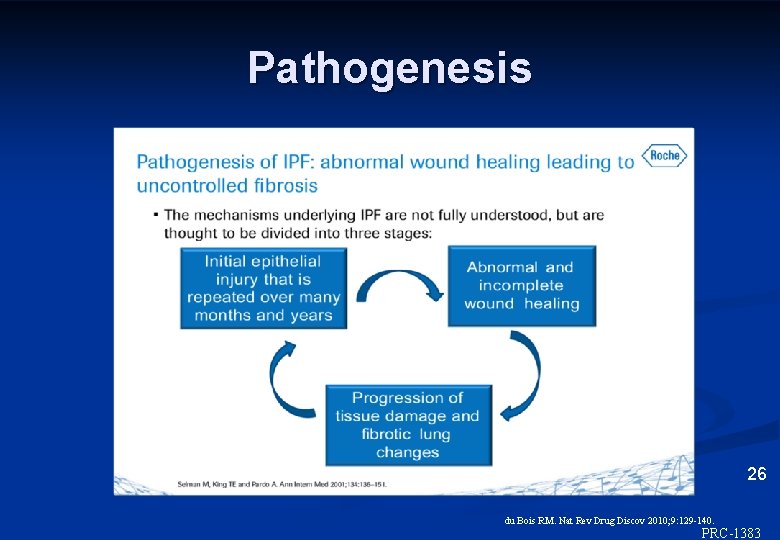
Pathogenesis 26 du Bois RM. Nat Rev Drug Discov 2010; 9: 129 -140. ©Inter. Mune, Inc. , 2012 PRC-1383

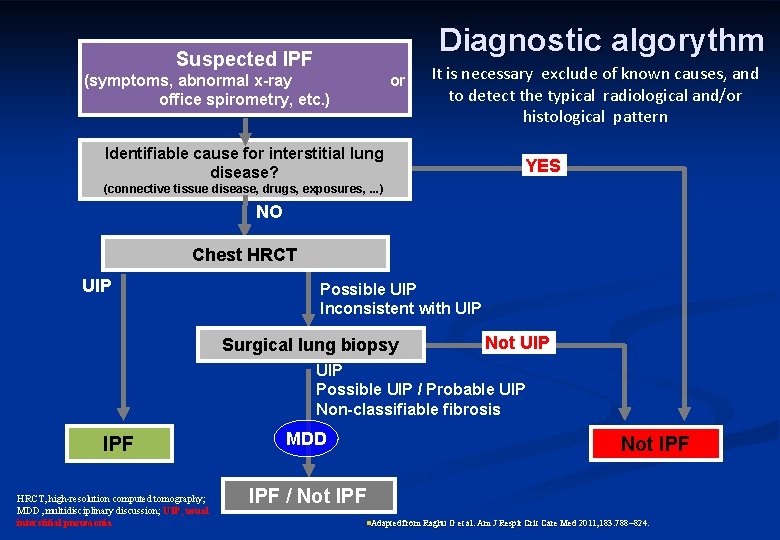
Diagnostic algorythm Suspected IPF (symptoms, abnormal x-ray office spirometry, etc. ) or It is necessary exclude of known causes, and to detect the typical radiological and/or histological pattern Identifiable cause for interstitial lung disease? YES (connective tissue disease, drugs, exposures, . . . ) NO Chest HRCT UIP Possible UIP Inconsistent with UIP Surgical lung biopsy Not UIP Possible UIP / Probable UIP Non-classifiable fibrosis IPF HRCT, high-resolution computed tomography; MDD, multidisciplinary discussion; UIP, usual interstitial pneumonia MDD Not IPF / Not IPF n. Adapted from Raghu G et al. Am J Respir Crit Care Med 2011; 183: 788 824.

Clinical features } } } Age >45 year Non-productive cough Slowly progressing dyspnoe Dry bilateral, inspiratory “Velcro -like” cracles Abnormal lung function – restrictive, impaired blood gases } Digital clubbing (25 -50%) } In late phase: cyanosis, cor pulmonale and oedema IPF is a severe, restrictive lung disease, limits on daily activities n 1. American Thoracic Society/European Respiratory Society. Am J Respir Crit Care Med 2002; 165: 277 304. n. NICE clinical guideline 163, June 2013. Available at n 2. Raghu G et al. Am J Respir Crit Care Med 2011; 183: 788 824; http: //www. nice. org. uk/guidance/cg 163. Accessed on 15 August 2014; n. Panos RJ et al. Am J Med 1990; 88: 396– 404; Image purchased from Science Photo Library.

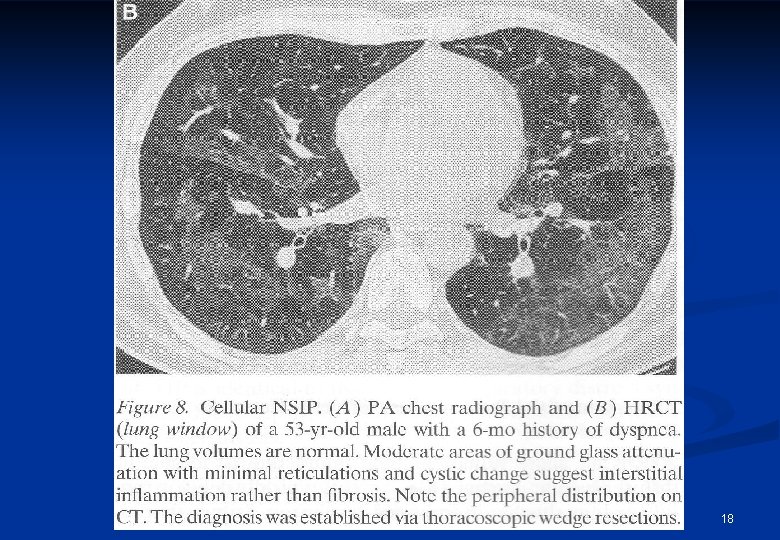
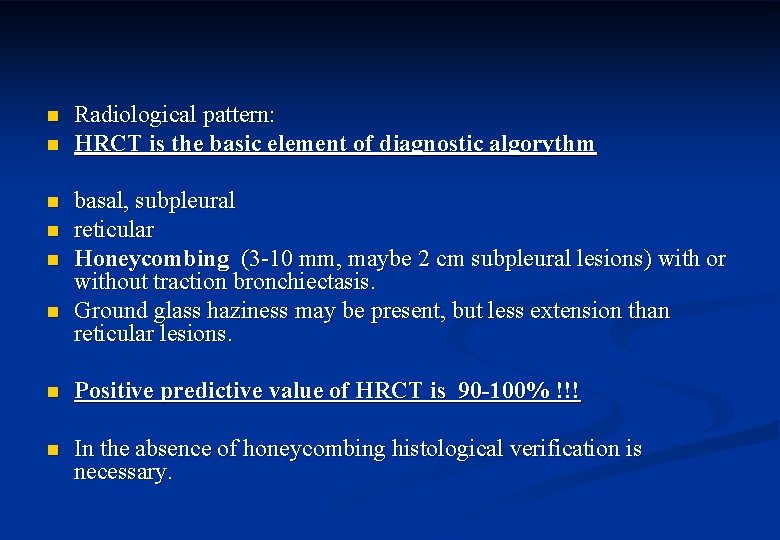
Usual interstitial pneumonia (UIP) proved by HRCT is the key of IPF diagnosis n HRCT is the basic element of diagnosis of IPF Diagnostc criteria of HRCT 1. Subpleural, basal predominance 2. Reticular abnormalities 3. „Honeycombing with or without bronchiectasis 4. If any of the above criteria is missing, we cannot diagnose UIP HRCT * †UIP ‡If pattern on HRCT is highly predictive of UIP pattern on surgical lung biopsy, with a positive predictive value of 90 100% not all four criteria are identified, then UIP pattern is classified as possible/inconsistent n. Raghu G et al. Am J Respir Crit Care Med 2011; 183: 788 824; n*Courtesy of Prof. M. Brauner, France. Reproduced with permission from: Valeyre D. Eur Resp Rev 2011; 20: 108 13.

n n n Radiological pattern: HRCT is the basic element of diagnostic algorythm basal, subpleural reticular Honeycombing (3 -10 mm, maybe 2 cm subpleural lesions) with or without traction bronchiectasis. Ground glass haziness may be present, but less extension than reticular lesions. n Positive predictive value of HRCT is 90 -100% !!! n In the absence of honeycombing histological verification is necessary.

Medication treatment n n n Previously IPF was consedered inflammatory disease, the medication was antiinflammaory treatment 1, 2 The inflammation has a subordinate rolek 1, 2 Fibrosis and the epithelial injury is more important, 2 Antifibrotic medications pirfenidon and nintedanib n 1. Selman M et al. Ann Intern Med 2001; 134: 136 151; ©Inter. Mune, Inc. , 2012 2. Günther A et al. Eur Respir Rev 2012; 21: 152 160.

IPF – Non drug treatments n Oxygen therapy n n In IPF with significant hypoxaemia 1 at rest Lung transplantation n Only a few patients with IPF 1, 2 1. ATS/ERS/JRS/ALAT. Am J Respir Crit Care Med 2011; 183: 788 -824 2. Meltzer EB, Noble PW. Orphanet J Rare Dis. 2008; 3: 8

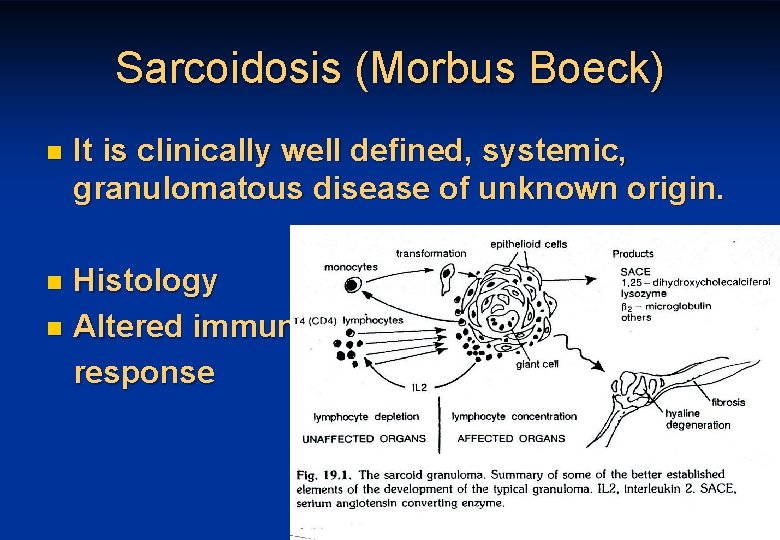
Sarcoidosis (Morbus Boeck) n It is clinically well defined, systemic, granulomatous disease of unknown origin. Histology n Altered immune response n 33

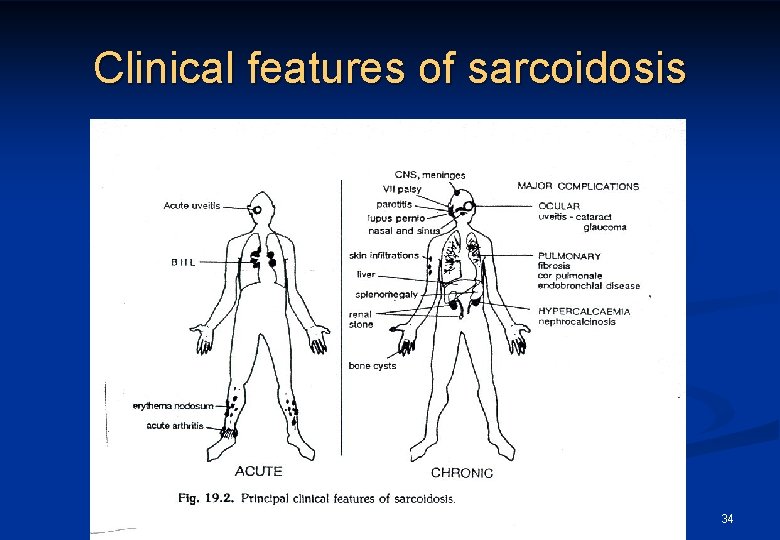
Clinical features of sarcoidosis 34

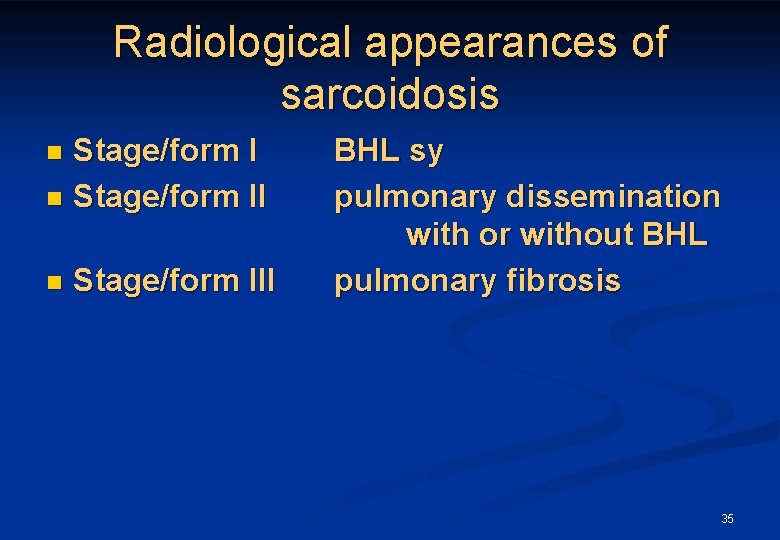
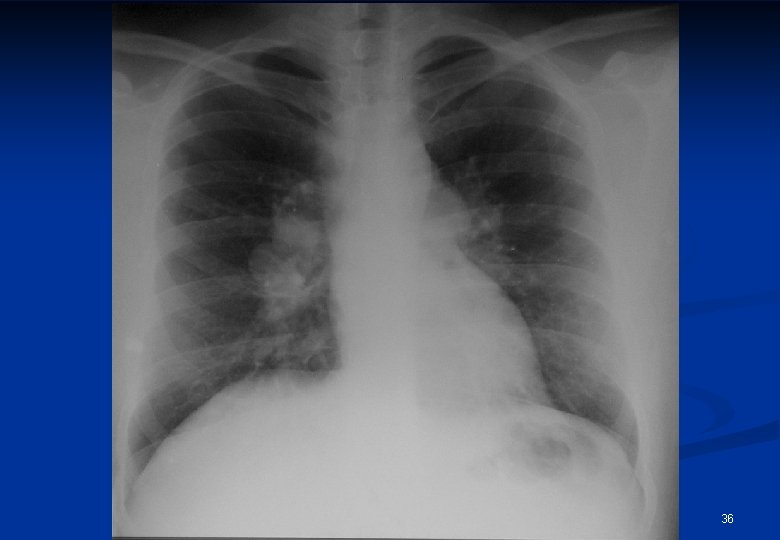
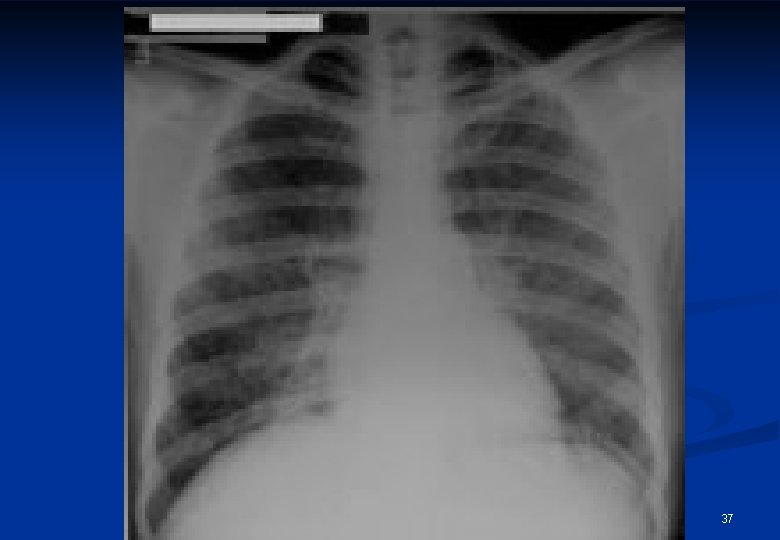
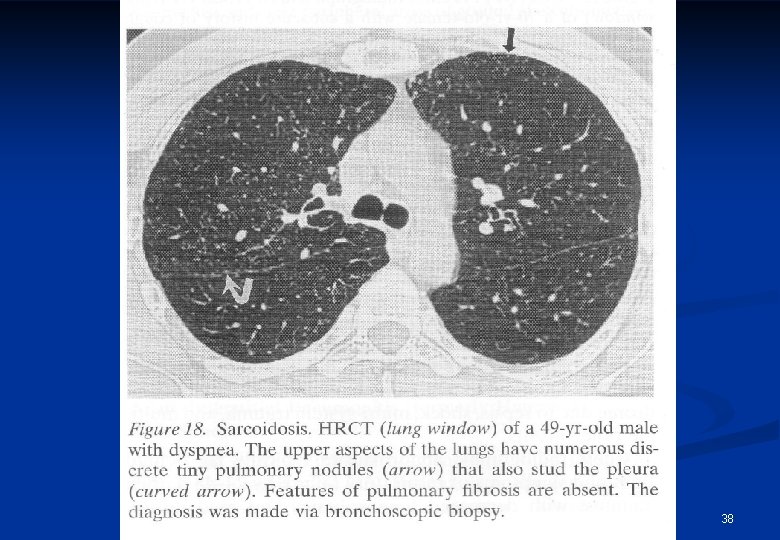
Radiological appearances of sarcoidosis Stage/form I n Stage/form II n n Stage/form III BHL sy pulmonary dissemination with or without BHL pulmonary fibrosis 35

36

37

38

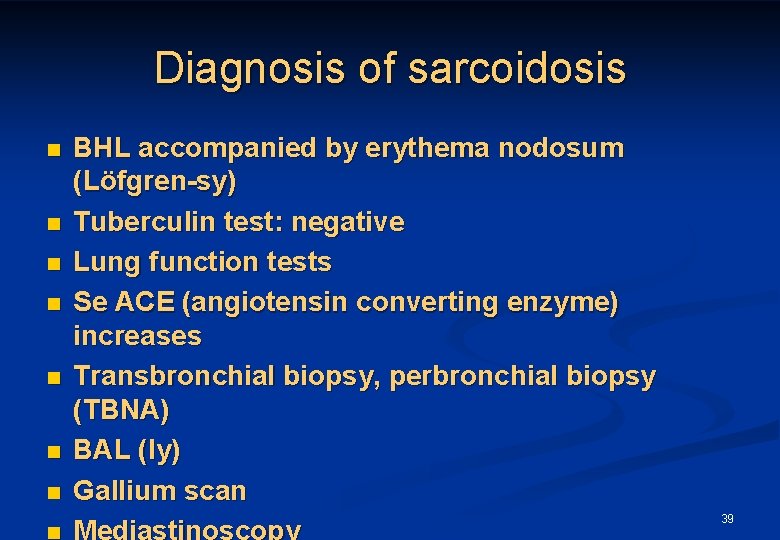
Diagnosis of sarcoidosis n n n n BHL accompanied by erythema nodosum (Löfgren-sy) Tuberculin test: negative Lung function tests Se ACE (angiotensin converting enzyme) increases Transbronchial biopsy, perbronchial biopsy (TBNA) BAL (ly) Gallium scan Mediastinoscopy 39

Treatment of sarcoidosis Stage I: no treatment is necessary n Indication of steroid treatment: n Progressive pulmonary disease n Severe uveitis n Hypercalcaemia n Neurological involvement n 40

Histiocytosis X (Langerhans) n n n This is a disorder of the mononuclear phagocyte system characterized by the accumulation of mononuclear phagocytes is various organs. In pediatric patients n Letterer-Siwe disease n Hand-Schüller-Christian disease In adults n Histiocytosis X n Eosinophilic granuloma 41

Clinical features of histiocytosis. X 20 -40 years of age (smoking) n Nonproductive cough n Dyspnea n Chest pain n Ptx – 10% of all cases n 42

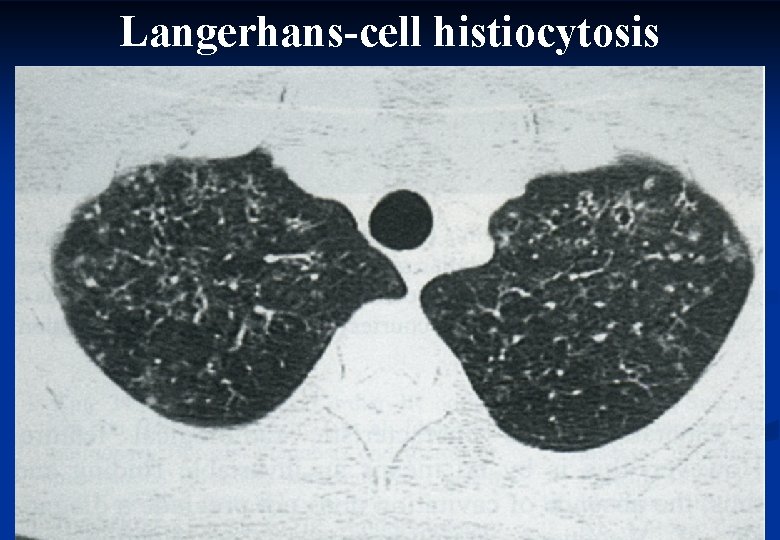
Characteristics of histiocytosis. X n X-ray changes n n n Lung function tests n n n Large number of mononuclear phagocytes Histology n n Mixed restrictive-obstructive pattern Decreased diffusing capacity BAL n n Reticulonodular shadow Small cystic spaces X bodies (Birbeck) in the cytoplasm There is no known treatment. Corticosteroids may be given. 43

Langerhans-cell histiocytosis 44

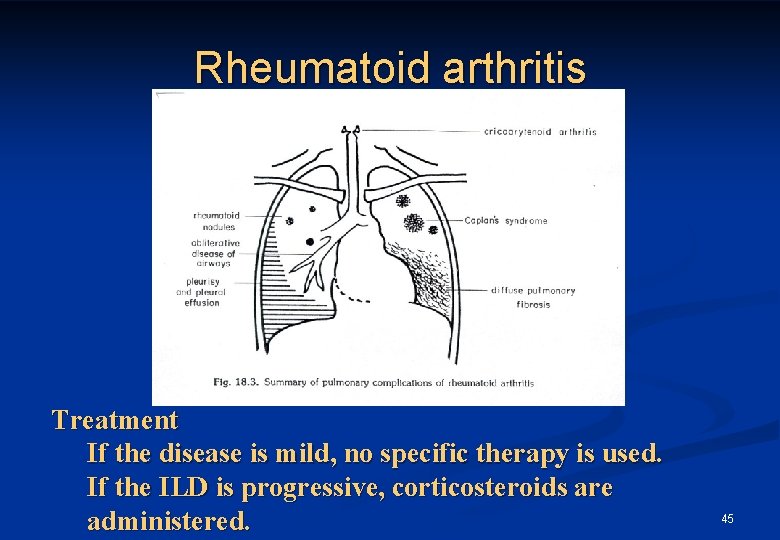
Rheumatoid arthritis Treatment If the disease is mild, no specific therapy is used. If the ILD is progressive, corticosteroids are administered. 45

Other immunological diseses SLE n Sjögren sy n Scleroderma n Bechterew n Dematomyositis, polymiositis n Periarteritis nodosa n Neurofibromatosis n 46

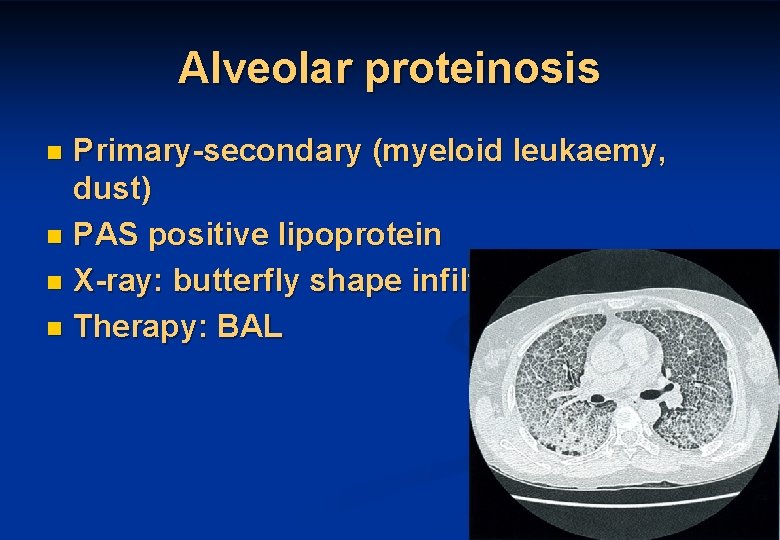
Alveolar proteinosis Primary-secondary (myeloid leukaemy, dust) n PAS positive lipoprotein n X-ray: butterfly shape infiltration n Therapy: BAL n 47

Eosinophylic pneumonias The eosinophylic pneumonias are characterized by eosinophilic pulmonary infiltrates and commonly peripherial blood eosinophylia. n Known and unknown etiology n 48

Eosinophylic pneumonias n n n Known etiology n Allergic bronchopulmonary aspergillosis (ABPA) n Parasitic infestations (ascaris, toxocara, etc) n Drug reactions (nitrofurantoin, sulphonamides) Idiopathic (unknown etiology) n Löffler’s sy n Benign, acute eosinophilic pneumonia (AEP) with migrating pulmonary infiltrates and minimal clinical manifestation. n Chr. eosinophilic pneumonia (CEP) n Symptoms: cough, sweats, fever, anorexia, weight loss, chills n X-ray: Peripherial infiltrates n Allergic granulomatosis of Churg and Strauss n Hypereosinophilic sy (HES) Therapy: corticosteroids 49

PNEUMOCONIOSIS Etiologic agents: dusts inhalation of inorganic metal dusts free silica coal dusts 50

HYPERSENSITIVE PNEUMONITIS (Extrinic allergic alveolitis) It is an immunologically induced inflammation of lung parenchyma involving alveolar walls and terminal airways secondary to repeated inhalation of a variety of organic dusts and other agents by susceptible host. Manifestations: Farmer’s lung (1932) – thermophylic actinomycetes Bird fancier’s breeder’s or handler’s lung Miller’s lung Bagassosis Byssinosis 51

Thank you for your attention! 52